Abstract
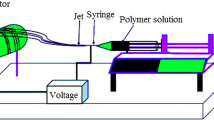
Effective recovery of uranium from wastewater has positive significance to environmental treatment and the development of the nuclear industry. Through electrospinning technology, the ternary layered double hydroxide (NiFeAl-LDHs), polyvinyl alcohol (PVA) and polyacrylic acid (PAA) was used as the precursor solution to produce NiFeAl-LDHs/PVA/PAA composite nanofibers, and it can adsorb uranium under weak acid conditions. The material structure and character of the prepared fiber were analyzed by SEM, FT-IR, XRD and XPS, besides the various factors on the adsorption of uranium by the fiber under static adsorption were studied. The adsorption process of NiFeAl-LDHs/PVA/PAA to U(VI) conformed to the pseudo-second-order model (R2 > 0.998). The maximum theoretical adsorption capacity of U(VI) on NiFeAl-LDHs/PVA/PAA was 203.32 mg/g at pH 6.0 calculated by the Langmuir model. The value of thermodynamic parameters showed that the adsorption process of uranium on NiFeAl-LDHs/PVA/PAA was endothermic and spontaneous. NiFeAl-LDHs/PVA/PAA can still effectively adsorb uranium after passing five adsorption–desorption cycle tests. Therefore, NiFeAl-LDHs/PVA/PAA was expected to be used in practical applications to treat uranium-containing wastewater.











Similar content being viewed by others
References
Wang S, Ran Y, Lu B, Li J, Kuang H, Gong L, Hao Y (2020) A Review of uranium-induced reproductive toxicity. Biol Trace Elem Res 196(1):204–213
Gao N, Huang Z, Liu H, Hou J, Liu X (2019) Advances on the toxicity of uranium to different organisms. Chemosphere 237:124548
Bjørklund G, Pivina L, Dadar M, Semenova Y, Rahman MM, Chirumbolo S, Aaseth J (2020) Depleted uranium and Gulf War Illness: updates and comments on possible mechanisms behind the syndrome. Environ Res 181:108927
Faa A, Gerosa C, Fanni D, Floris G, Eyken PV, Lachowicz JI, Nurchi VM (2018) Depleted uranium and human health. Curr Med Chem 25(1):49–64
Bjorklund G, Semenova Y, Pivina L, Dadar M, Rahman MM, Aaseth J, Chirumbolo S (2020) Uranium in drinking water: a public health threat. Arch Toxicol 94(5):1551–1560
Lourenco J, Marques S, Carvalho FP, Oliveira J, Malta M, Santos M, Goncalves F, Pereira R, Mendo S (2017) Uranium mining wastes: the use of the fish embryo acute toxicity test (FET) test to evaluate toxicity and risk of environmental discharge. Sci Total Environ 605:391–404
Ouyang J, Liu Z, Zhang L, Wang Y, Zhou L (2020) Analysis of influencing factors of heavy metals pollution in farmland-rice system around a uranium tailings dam. Process Saf Environ 139:124–132
Ouyang J, Liu Z, Ye T, Zhang L (2019) Uranium pollution status and speciation analysis in the farmland-rice system around a uranium tailings mine in southeastern China. J Radioanal Nucl Ch 322(2):1011–1022
Selvakumar R, Ramadoss G, Menon MP, Rajendran K, Thavamani P, Naidu R, Megharaj M (2018) Challenges and complexities in remediation of uranium contaminated soils: a review. J Environ Radioactiv 192:592–603
Sun Y, Li Y (2021) Application of surface complexation modeling on adsorption of uranium at water-solid interface: A review. Environ Pollut 278:116861
Xue G, Yurun F, Li M, Dezhi G, Jie J, Jincheng Y, Haibin S, Hongyu G, Yujun Z (2017) Phosphoryl functionalized mesoporous silica for uranium adsorption. Appl Surf Sci 402:53–60
Rosenberg E, Pinson G, Tsosie R, Tutu H, Cukrowska E (2016) Uranium remediation by ion exchange and sorption methods: a critical review various types of solid phase sorbents are studied and evaluated. Johnson Matthey Tech 60(1):59–77
Li L, Zhao Y, Jin Y, Linghu W, Chen C, Asiri AM, Marwani HM, Sheng G (2019) Efficient scavenging of uranium (VI) using porous hexagonal boron nitride by a combined process of surface adsorption and induced precipitation crystallization. J Radioanal Nucl Ch 321(3):1035–1044
Geran S, Chamelot P, Serp J, Gibilaro M, Massot L (2020) Electrochemistry of uranium in molten LiCl-LiF. Electrochim Acta 355:136784
He S, Yang Z, Cui X, Zhang X, Niu X (2020) Fabrication of the novel Ag-doped SnS2@ InVO4 composite with high adsorption-photocatalysis for the removal of uranium (VI). Chemosphere 260:127548
Li P, Wang J, Wang Y, Liang J, He B, Pan D, Fan Q, Wang X (2019) Photoconversion of U(VI) by TiO2: an efficient strategy for seawater uranium extraction. Chem Eng J 365:231–241
Wu J, Tian K, Wang J (2018) Adsorption of uranium (VI) by amidoxime modified multiwalled carbon nanotubes. Prog Nucl Energ 106:79–86
Lyu P, Wang G, Cao Y, Wang B, Deng N (2021) Phosphorus-modified biochar cross-linked Mg–Al layered double-hydroxide composite for immobilizing uranium in mining contaminated soil. Chemosphere 276:130116
Tatarchuk T, Shyichuk A, Mironyuk I, Naushad M (2019) A review on removal of uranium (VI) ions using titanium dioxide based sorbents. J Mol Liquids 293:111563
Yang W, Pan Q, Song S, Zhang H (2019) Metal–organic framework-based materials for the recovery of uranium from aqueous solutions. Inorg Chem Front 6(8):1924–1937
Wu H, Chi F, Zhang S, Wen J, Xiong J, Hu S (2019) Control of pore chemistry in metal-organic frameworks for selective uranium extraction from seawater. Microporous and Mesoporous Mater 288:109567
Gu P, Zhang S, Li X, Wang X, Wen T, Jehan R, Alsaedi A, Hayat T, Wang X (2018) Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ Pollut 240:493–505
Yu S, Wang X, Liu Y, Chen Z, Wu Y, Liu Y, Pang H, Song G, Chen J, Wang X (2019) Efficient removal of uranium(VI) by layered double hydroxides supported nanoscale zero-valent iron: A combined experimental and spectroscopic studies. Chem Eng J 365:51–59
Naeem H, Bhatti HN, Sadaf S, Iqbal M (2017) Uranium remediation using modified Vigna radiata waste biomass. Appl Radiat Isotopes 123:94–101
Yi Z, Liu J, Zeng R, Liu X, Long J, Huang B (2020) Removal of uranium(VI) from aqueous solution by Camellia oleifera shell-based activated carbon: adsorption equilibrium, kinetics, and thermodynamics. Water Sci Technol 82(11):2592–2602
Singhal P, Vats BG, Pulhani V (2020) Magnetic nanoparticles for the recovery of uranium from sea water: challenges involved from research to development. J Ind Eng Chem 90:17–35
Calì E, Qi J, Preedy O, Chen S, Boldrin D, Branford WR, Vandeperre L, Ryan MP (2018) Functionalised magnetic nanoparticles for uranium adsorption with ultra-high capacity and selectivity. J Mater Chem A 6(7):3063–3073
Zhu F, Zheng YM, Zhang BG, Dai YR (2021) A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J Hazard Mater 401:123608
Bode-Aluko CA, Pereao O, Ndayambaje G, Petrik L (2016) Adsorption of Toxic Metals on Modified Polyacrylonitrile Nanofibres: A Review. Water Air Soil Poll 228(1):1–11
Hu X, Wang Y, Yang JO, Li Y, Wu P, Zhang H, Yuan D, Liu Y, Wu Z, Liu Z (2020) Synthesis of graphene oxide nanoribbons/chitosan composite membranes for the removal of uranium from aqueous solutions. Front Chem Sci Eng 14(6):1029–1038
Christou C, Philippou K, Krasia-Christoforou T, Pashalidis I (2019) Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers. Carbohyd Polym 219:298–305
Gebru KA, Das C (2017) Removal of Pb (II) and Cu (II) ions from wastewater using composite electrospun cellulose acetate/titanium oxide (TiO2) adsorbent. J Water Process Eng 16:1–13
Wang Y, Zhang Y, Li Q, Li Y, Cao L, Li W (2020) Amidoximated cellulose fiber membrane for uranium extraction from simulated seawater. Carbohyd Polym 245:116627
Wang F, Song Y, Liang S, Yu Y, Liang J, Jiang M (2021) Polyamidoxime nanoparticles/polyvinyl alcohol composite chelating nanofibers prepared by centrifugal spinning for uranium extraction. React Funct Polym 159:104812
Talebi M, Abbasizadeh S, Keshtkar AR (2017) Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process Saf Environ 109:340–356
Xie J, Lv R, Peng H, Fan J, Liu Y (2020) Phosphate functionalized poly(vinyl alcohol)/poly(acrylic acid) (PVA/PAA): an electrospinning nanofiber for uranium separation. J Radioanal Nucl Ch 326(1):475–486
Zulfikar MA, Maulina D, Nasir M, Handayani N, Handajani M (2020) Removal of methylene blue from aqueous solution using poly (acrylic acid)/SiO2 and functionalized poly (acrylic acid)/SiO2 composite nanofibers. Environ Nanotechnol Monitoring Manag 14:100381
Park JA, Kang JK, Lee SC, Kim SB (2017) Electrospun poly(acrylic acid)/poly(vinyl alcohol) nanofibrous adsorbents for Cu(II) removal from industrial plating wastewater. RSC Adv 7(29):18075–18084
Xiao SL, Luo XY, Peng QY, Deb H (2016) Effective removal of calcium ions from simulated hard water using electrospun polyelectrolyte nanofibrous mats. Fiber Polym 17(9):1428–1437
Zhang SJ, Shi QT, Christodoulatos C, Korfiatis G, Meng XG (2019) Adsorptive filtration of lead by electrospun PVA/PAA nanofiber membranes in a fixed-bed column. Chem Eng J 370:1262–1273
Kim J, Kang T, Kim H, Shin HJ, Oh S-G (2019) Preparation of PVA/PAA nanofibers containing thiol-modified silica particles by electrospinning as an eco-friendly Cu(II) adsorbent. J Ind Eng Chem 77:273–279
Xiao SL, Ma H, Shen MW, Wang SY, Huang QG, Shi XY (2011) Excellent copper(II) removal using zero-valent iron nanoparticle-immobilized hybrid electrospun polymer nanofibrous mats. Colloid Surface A 381(1–3):48–54
Song S, Yin L, Wang XX, Liu L, Huang SY, Zhang R, Wen T, Yu SJ, Fu D, Hayat T, Wang XK (2018) Interaction of U(VI) with ternary layered double hydroxides by combined batch experiments and spectroscopy study. Chem Eng J 338:579–590
Wang PY, Yin L, Wang XX, Zhao GX, Yu SJ, Song G, Xie J, Alsaedi A, Hayat T, Wang XK (2018) L-cysteine intercalated layered double hydroxide for highly efficient capture of U(VI) from aqueous solutions. J Environ Manage 217:468–477
Karami Z, Jouyandeh M, Ali JA, Ganjali MR, Aghazadeh M, Maadani M, Saeb MR (2019) Development of Mg-Zn-Al-CO3 ternary LDH and its curability in epoxy/amine system. Prog Org Coat 136:105264
Zou YD, Wang XX, Wu F, Yu SJ, Hu YZ, Song WC, Liu YH, Wang HQ, Hayat T, Wang XK (2017) Controllable synthesis of Ca-Mg-Al layered double hydroxides and calcined layered double oxides for the efficient removal of U(VI) from wastewater solutions. ACS Sustain Chem Eng 5(1):1173–1185
Butenko E, Bish D, Abrosimova G, Kapustin A (2013) Comparison of sorption properties of natural and synthetic takovites, Ni6Al2(OH)16CO3·4H2O. Építőanyag: JSBCM 65(4):97–101
Valeikiene L, Roshchina M, Grigoraviciute-Puroniene I, Prozorovich V, Zarkov A, Ivanets A, Kareiva A (2020) On the Reconstruction Peculiarities of Sol-Gel Derived Mg2-xMx/Al1(M = Ca, Sr, Ba) Layered Double Hydroxides. Curr Comput-Aided Drug Des 10(6):470
Li Y, He H, Liu Z, Lai Z, Wang Y (2021) A facile method for preparing three-dimensional graphene nanoribbons aerogel for uranium (VI) and thorium (IV) adsorption. J Radioanal Nuclear Chem 328(1):289–298
Aslani CK, Amik O (2021) Active Carbon/PAN composite adsorbent for uranium removal: Modeling adsorption isotherm data, thermodynamic and kinetic studies. Appl Radiation and Isotopes 168:109474
Hu R, Xiao J, Wang T, Chen G, Chen L, Tian X (2020) Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem Eng J 379:122388
Li P, Chen P, Liu Z, Nie S, Wang X, Wang G, Wang L (2020) Highly efficient elimination of uranium from wastewater with facilely synthesized Mg-Fe layered double hydroxides: Optimum preparation conditions and adsorption kinetics. Anna Nuclear Energy 140:107140
Huang Y, Zheng H, Li H, Zhao C, Zhao R, Li S (2020) Highly selective uranium adsorption on 2-phosphonobutane-1, 2, 4-tricarboxylic acid-decorated chitosan-coated magnetic silica nanoparticles. Chem Eng J 388:124349
Liu L, Lin XY, Li MS, Chu HH, Wang HY, Xie Y, Du ZC, Liu MJ, Liang LL, Gong HY, Zhou J, Li ZG, Luo XG (2021) Microwave-assisted hydrothermal synthesis of carbon doped with phosphorus for uranium(VI) adsorption. J Radioanal Nucl Ch 327(1):73–89
Wang Y, Li YX, Li L, Kong FG, Lin S, Wang ZQ, Li WL (2020) Preparation of three-dimensional fiber-network chitosan films for the efficient treatment of uranium-contaminated effluents. Water Sci Technol 81(1):52–61
Yang S, Li Q, Chen L, Chen Z, Hu B, Wang H, Wang X (2020) Synergistic removal and reduction of U (VI) and Cr (VI) by Fe3S4 micro-crystal. Chem Eng J 385:123909
Alahabadi A, Singh P, Raizada P, Anastopoulos I, Sivamani S, Dotto GL, Hosseini-Bandegharaei A (2020) Activated carbon from wood wastes for the removal of uranium and thorium ions through modification with mineral acid. Colloids Surf A Physicochem Eng Aspects 607:125516
Acknowledgements
The present work was financially supported by National Natural Science Foundation of China (21866003, 22066001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, J., Dai, Y., Wang, Y. et al. Facile immobilization of NiFeAl-LDHs into electrospun poly(vinyl alcohol)/poly(acrylic acid) nanofibers for uranium adsorption. J Radioanal Nucl Chem 329, 1103–1117 (2021). https://doi.org/10.1007/s10967-021-07860-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07860-3