Abstract
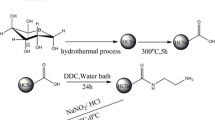
The phosphorylated hydrothermal carbon spheres (HCS-PO4) were developed by functionalizing hydrothermal carbon spheres (HCS) with o-phosphoethanolamine, and the structure and textural property were characterized by SEM and FT-IR. The parameters that affect the uranium(VI) sorption, such as solution pH, initial U(VI) concentration, contact time, and temperature, had been investigated. The HCS-PO4 showed the highest uranium sorption capacity at initial pH 6.0 and contact time of 120 min. The adsorption kinetics was better described by the pseudo-second-order model, and the adsorption process could be well defined by the Langmuir isotherm and the maximum monolayer adsorption capacity increased from 80.00 to 434.78 mg/g after phosphorylation. The thermodynamic parameters, ∆G° (298 K), ∆H° and ∆S°, demonstrated shown that the sorption process of U(VI) onto HCS-PO4 was feasible, spontaneous and endothermic in nature. The spent HCS-PO4 could be effectively regenerated by 0.1 mol/L EDTA solution for the removal and recovery of U(VI) and reused for ten cycles at least. Selective adsorption studies showed that the HCS-PO4 could selectively remove U(VI), and the selectivity coefficients of HCS in the presence of co-existing ions, Mg(II), Na(I), Zn(II), Mn(II),Co(II), Ni(II), Sr(II), Cs(I) and Hg(II) improved after functionalization.














Similar content being viewed by others
References
Mudd GM (2008) Radon releases from Australian uranium mining and milling projects: assessing the UNSCEAR approach. J Environ Radioact 99:288–315
Rao TP, Metilda P, Gladis JM (2006) Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination: an overview. Talanta 68:1047–1064
Anirudhan T, Bringle C, Rijith S (2010) Removal of uranium(VI) from aqueous solutions and nuclear industry effluents using humic acid-immobilized zirconium-pillared clay. J Environ Radioact 101:267–276
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) An assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manag 92:2504–2512
Takeda T, Saito K, Uezu K, Furusaki S, Sugo T, Okamoto J (1991) Adsorption and elution in hollow-fiber-packed bed for recovery of uranium from seawater. Ind Eng Chem Res 30:185–190
Coleman SJ, Coronado PR, Maxwell RS, Reynolds JG (2003) Granulated activated carbon modified with hydrophobic silica aerogel-potential composite materials for the removal of uranium from aqueous solutions. Environ Sci Technol 37:2286–2290
Zhao Y, Liu C, Feng M, Chen Z, Li S, Tian G, Wang L, Huang J, Li S (2010) Solid phase extraction of uranium(VI) onto benzoylthiourea-anchored activated carbon. J Hazard Mater 176:119–124
Schierz A, Zänker H (2009) Aqueous suspensions of carbon nanotubes: surface oxidation, colloidal stability and uranium sorption. Environ Pollut 157:1088–1094
Shao D, Jiang Z, Wang X, Li J, Meng Y (2009) Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO2 2+ from aqueous solution. J Phys Chem B 113:860–864
Xu Y, Zondlo JW, Finklea HO, Brennsteiner A (2000) Electrosorption of uranium on carbon fibers as a means of environmental remediation. Fuel Process Technol 68:189–208
Starvin A, Rao TP (2004) Solid phase extractive preconcentration of uranium(VI) onto diarylazobisphenol modified activated carbon. Talanta 63:225–232
Sun X, Li Y (2004) Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew Chem Int Ed 43:597–601
Wang Q, Li H, Chen L, Huang X (2001) Monodispersed hard carbon spherules with uniform nanopores. Carbon 39:2211–2214
Mi Y, Hu W, Dan Y, Liu Y (2008) Synthesis of carbon micro-spheres by a glucose hydrothermal method. Mater Lett 62:1194–1196
Zheng M, Cao J, Chang X, Wang J, Liu J, Ma X (2006) Preparation of oxide hollow spheres by colloidal carbon spheres. Mater Lett 60:2991–2993
Zhuang Z, Yang Z (2009) Preparation and characterization of colloidal carbon sphere/rigid polyurethane foam composites. J Appl Polym Sci 114:3863–3869
Yao C, Shin Y, Wang LQ, Windisch CF, Samuels WD, Arey BW, Wang C, Risen WM, Exarhos GJ (2007) Hydrothermal dehydration of aqueous fructose solutions in a closed system. J Phys Chem C 111:15141–15145
Ratchahat S, Viriya-empikul N, Faungnawakij K, Charinpanitkul T, Soottitantawat A (2010) Synthesis of carbon microspheres from starch by hydrothermal process. Sci J UBU 1:40–45
Sevilla M, Fuertes AB (2009) The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 47:2281–2289
Titirici M-M, Antonietti M (2010) Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem Soc Rev 39:103–116
Geng J, Ma L, Wang H, Liu J, Bai C, Song Q, Li J, Hou M, Li S (2012) Amidoxime-grafted hydrothermal carbon microspheres for highly selective separation of uranium. J Nanosci Nanotechnol 12:7354–7363
Song Q, Ma L, Liu J, Bai C, Geng J, Wang H, Li B, Wang L, Li S (2012) Preparation and adsorption performance of 5-azacytosine-functionalized hydrothermal carbon for selective solid-phase extraction of uranium. J Colloid Interface Sci 386:291–299
Wang H, Ma L, Cao K, Geng J, Liu J, Song Q, Yang X, Li S (2012) Selective solid-phase extraction of uranium by salicylideneimine-functionalized hydrothermal carbon. J Hazard Mater 229–230:321–330
Liu Y, Wang Y, Zhang Z, Cao X, Nie W, Li Q, Hua Q (2013) Removal of uranium from aqueous solution by a low cost and high-efficient adsorbent. Appl Surf Sci 273:68–74
Zhang Z, Zhou Z, Cao X, Liu Y, Xiong G, Liang P (2013) Removal of uranium(VI) from aqueous solutions by new phosphorus-containing carbon spheres synthesized via one-step hydrothermal carbonization of glucose in the presence of phosphoric acid. J Radioanal Nucl Chem 299:1479–1487
Kabay N, Demircioglu M, Yayli S, Günay E, Yüksel M, Saglam M, Streat M (1998) Recovery of uranium from phosphoric acid solutions using chelating ion-exchange resins. Ind Eng Chem Res 37:1983–1990
Chen Z, Ma L, Li S, Geng J, Song Q, Liu J, Wang C, Wang H, Li J, Qin Z (2011) Simple approach to carboxyl-rich materials through low-temperature heat treatment of hydrothermal carbon in air. Appl Surf Sci 257:8686–8691
Nie W, Zhang Z, Cao X, Liu Y, Liang P (2013) Sorption study of uranium from aqueous solution on ordered mesoporous carbon CMK-3. J Radioanal Nucl Chem 295:663–670
Fanning PE, Vannice MA (1993) A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 31:721–730
Edwards JC, Thiel CY, Benac B, Knifton JF (1998) Solid-state NMR and FT-IR investigation of 12-tungstophosphoric acid on TiO2. Catal Lett 51:77–83
Preetha CR, Gladis JM, Rao TP, Venkateswaran G (2006) Removal of toxic uranium from synthetic nuclear power reactor effluents using uranyl ion imprinted polymer particles. Environ Sci Technol 40:3070–3074
Bayramoğlu G, Çelik G, Arica MY (2006) Studies on accumulation of uranium by fungus Lentinus sajor-caju. J Hazard Mater 136:345–353
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Psareva T, Zakutevskyy O, Chubar N, Strelko V, Shaposhnikova T, Carvalho JR, Correia M (2005) Uranium sorption on cork biomass. Colloids Surf A 252:231–236
Anirudhan TS, Rijith S, Tharun AR (2010) Adsorptive removal of thorium(IV) from aqueous solutions using poly (methacrylic acid)-grafted chitosan/bentonite composite matrix: process design and equilibrium studies. Colloids Surf A 368:13–22
Aytas S, Yurtlu M, Donat R (2009) Adsorption characteristic of U(VI) ion onto thermally activated bentonite. J Hazard Mater 172:667–674
Anirudhan TS, Radhakrishnan PG (2009) Improved performance of a biomaterial-based cation exchanger for the adsorption of uranium(VI) from water and nuclear industry wastewater. J Environ Radioact 100:250–257
Hazer O, Kartal Ş (2010) Use of amidoximated hydrogel for removal and recovery of U(VI) ion from water samples. Talanta 82:1974–1979
Parab H, Joshi S, Shenoy N, Verma R, Lali A (2012) Uranium removal of aqueous solution by coir pith: equilibrium and kinetic studies. Bioresour Technol 96:1241–1248
Aytas S, Turkozu DA, Gok C (2011) Biosorption of uranium(VI) by bi-functionalized low cost biocomposite adsorbent. Desalination 280:354–362
Anirudhan T, Divya L, Suchithra P (2009) Kinetic and equilibrium characterization of uranium(VI) adsorption onto carboxylate-functionalized poly (hydroxyethylmethacrylate)-grafted lignocellulosics. J Environ Manag 90:549–560
Gürboğa G, Tel H (2005) Preparation of TiO2–SiO2 mixed gel spheres for strontium adsorption. J Hazard Mater 120:135–142
Donia A, Atia A, Moussa E, Elsherif A, Abdelmagied M (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95:183–189
Acknowledgments
We gratefully acknowledge the financial support provided by National Natural Science Foundation of China (Grant Nos. 21101024, 21201033,21301028), National Undergraduate Training Programs for Innovation and Entrepreneurship (Grant No. 201210405006), Key Project of Chinese Ministry of Education (Grant No. 211086), the Young Scientists Training Program of Jiangxi Province (Grant No. 20122BCB23023), Natural Science Foundation of Jiangxi Province (Grant Nos. 20114BAB203002, 20122BAB203012, 20132BAB203027), China Postdoctoral Science Foundation (Grant No. 20110490857), and Project of Jiangxi Provincial Department of Education (Grant No. GJJ13452) and Open Project Foundation of Fundamental Science on Radioactive Geology and Exploration Technology Laboratory (East China Institute of Technology) (Grant No. RGET1311).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XF., Liu, YH., Zhou, ZW. et al. Adsorptive removal of U(VI) from aqueous solution by hydrothermal carbon spheres with phosphate group. J Radioanal Nucl Chem 300, 1235–1244 (2014). https://doi.org/10.1007/s10967-014-3081-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3081-6