Abstract
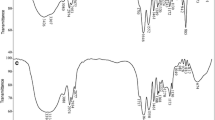
Polyacrylic acid hydrogel was synthesized by Free Radical polymerization and characterized by means of FTIR. The FTIR results show that the carboxylic groups in the complexes coordinated to the metal ions in the form of two dentate. The effects of contact time, solid/liquid ratio, pH value, and initial concentration on the adsorption of UO2 2+ ions onto polyacrylic acid were investigated. The adsorption of UO2 2+ ions was highly dependent on the initial pH of metal ions solution and initial metal ions concentration. The adsorption kinetic data indicated that the chemical adsorption was the swiftness processes, the adsorption equilibrium could be achieved within 15 min. And there are very good correlation coefficients of linearized equations for Freundlich model, which indicated that the sorption isotherm of the hydrogel for UO2 2+ can be fitted to the Freundlich model. It was found that the maximum adsorption quantity of UO2 2+ was 1,179 mg/g. After five times of repeated tests for the hydrogel it still remained its excellent adsorption.







Similar content being viewed by others
References
Prasada Rao T, Metilda P, Gladis JM (2006) Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination-an overview. Talanta 68:1047–1064
Eisenbud M, Gesell T (1997) Environmental radioactivity from natural, industrial, and military sources. Academic Press, Boston
Choppin GR, Morgenstern A (2000) Radionuclide separations in radioactive wastes disposal. J Radioanal Nucl Chem 243:45–51
Xie SB, Yang J, Chen C, Zhang XJ, Wang QL, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radiact 99:126–133
Van Horn JD, Huang H (2006) Uranium(VI) bio-coordination chemistry from biochemical, solution and protein structural data. Coord Chem Rev 250:765–775
Mellah AS, Chegrouche Barkat M (2007) The precipitation of ammonium uranyl carbonate (AUC): thermodynamic and kinetic investigations. Hydrometallurgy 85:163–171
Gu B, Ku Y, Brown GM (2005) Sorption and desorption of perchlorate and U(VI) by strong-base anion-exchange resins. Environ Sci Technol 39(3):901–907
Kanekar AS, Pathak PN, Mohapatra PK, Manchanda VK (2010) Comparative extraction efficiencies of tri-n-butyl phosphate and N,N-dihexyloctanamide for uranium recovery using supercritical CO2. J Radioanal Nucl Chem 283:789–796
Kuhu AT (1972) Electrochemistry of cleaner environments. Plenum Press, New York
Dietz ML, Philip HE, Sajdak LR, Chiarizia R (2001) An improved extraction chromatographic resin for the separation of uranium from acidic nitrate media. Talanta 54(6):1173–1184
Jung Y, Kim S, Park S, Kim JM (2008) Application of polymer-modified nanoporous silica to adsorbents of uranyl ions. Colloids Surf A 313–314:162–166
Tsuruta T (2011) Biosorption of uranium for environmental applications using bacteria isolated from the uranium deposits. In: Microbes and Microbial Technology, Chap 11, pp 267–281
Unuabonah EI, Adebowal KO, Olu-owolabi BI, Yang LZ, Kong LX (2008) Adsorption of Pb (ll) and Cd (ll) from aqueous solutions onto sodium tetraborate-modified Kaolinite clay: equilibrium and thermodynamic studies. Hydrometallurgy 93:1–9
Wang CC, Chang CY, Chen CY (2001) Study on metal ion adsorption of bifunctional chelating/ion-exchange resins. Macromol Chem Phys 202(6):882–890
Biswas M, Mukterjee A (1994) Synthesis and evaluation of metal-containing polymers. Adv Polym Sci 115:89–123
Wang Q, Mynar JL, Yoshida M, Lee E, Lee M, Okuro K, Kinbara K, Aida T (2010) High–water–content hydrogel by mixing clay and dendritic. Mol Glue 463:339–343
Özeroğlu C, Doğan E, Keçeli G (2011) Investigation of Cs(I) adsorption on densely crosslinked poly(sodium methacrylate) from aqueous solutions. J Radioanal Nucl Chem 289:577–586
Wang Q, Xie X, Zhang X, Zhang J, Wang A (2010) Preparation and swelling properties of pH-sensitive composite hydrogel beads based on chitosan-g-poly (acrylic acid)/vermiculite and sodium alginate for diclofenac controlled release. Int J Biol Macromol 46:356–362
Paulino AT, Guilherme MR, Reis AV, Campese GM, Muniz EC, Nozaki J (2006) Removal of methylene blue dye from an aqueous media using superabsorbent hydrogel supported on modified polysaccharide. J Colloid Interface Sci 301:55–62
Yetimoğlu EK, Kahraman MV, Bayramoğlu G, Ercan O, Apohan NK (2009) Sulfathiazole-based novel UV-cured hydrogel sorbents for mercury removal from aqueous solutions. Radiat Phys Chem 78:1800–1806
Zheng Y, Zhang J, Wang A (2009) Fast removal of ammonium nitrogen from aqueous solution using chitosan-g-poly(acrylic acid)/attapulgite composite. Chem Eng J 155:215–222
Liu Y, Zheng Y, Wang A (2010) Enhanced adsorption of methylene blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J Environ Sci 22:486–493
O’Connor SM, Gehrke SH (1997) Synthesis and characterization of thermally-responsive hydroxypropyl methylcellulose gel spheres. J Appl Polym Sci 66:1279–1290
Li N, Bai RB (2005) A novel amine-shielded surface cross-linking of chitosan hydrogel beads for enhanced metal adsorption performance. Ind Eng Chem Res 44:6692–6700
Yan WL, Bai RB (2005) Adsorption of lead and humic acid on chitosan hydrogel beads. Water Res 39:688–698
Wei M, Liao JL, Liu N, Zhang D, Kang HJ, Yang YY, Yong Y, Jin JN (2007) Interaction between uranium and humic acid (I): adsorption behaviors of U(VI) in soil humic acids. Nucl Sci Tech 18:287–293
Wang GH, Liu JS, Wang XG, Xie ZY, Deng NS (2009) Adsorption of uranium(VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Liu TH, Fang J, Zhang Y, Zeng ZZ (2008) The effect of salt and pH on the phase transition behaviors of pH and temperature-responsive poly(N,N-dimethylacrylamide-co-methylacrylic acid). Macromol Res 16(8):670–675
Kakihana M, Nagumo T (1987) Coordination structures for uranyl carboxylate complexes in aqueous solution studied by IR and carbon-13 NMR spectra. J Phys Chem 91:6128–6136
Liu TH, Duan GH, Zeng ZZ (2009) Synthesis and characterization of cerium, thorium, and uranyl complexes with (E)-4-(4-methoxyphenoxy)-4-oxobut-2-enoic acid. J Coord Chem 62(13):2203–2211
Nakamoto K (1986) Infrared spectra and raman spectra of inorganic and coordination compounds. Wiley, New York
Kuppusamy K, Sivasankar BN, Govindarajan S (1996) Preparation and thermal reactivity of hydrazinium uranyl carboxylates. Thermochim Acta 274:139–148
Rivas BL, Maturana HA, Ocampa X, Peric IM (1995) Adsorption behavior of Cu2+ and UO2 2+ ions on crosslinked poly[2,2-bis(acrylamido)acetic acid]. J Appl Polym Sci 58:2201–2205
Yang ZY, Yang RD, Li FS, Yu KB (2000) Crystal structure and antitumor activity of some rare earth metal complexes with Schiff base. Polyhedron 19:2599–2604
Riaz Q (2012) A study of the factors affecting the adsorption of cobalt ions onto Pakistani coal powder from solutions. J Radioanal Nucl Chem. doi:10.1007/s10967-012-2189-9
Lu S, Guo Z, Zhang C, Zhang S (2011) Sorption of Th(IV) on MX-80 bentonite: effect of pH and modeling. J Radioanal Nucl Chem 287:621–628
Ma JH, Xu YJ, Fan B, Liang BR (2007) Preparation and characterization of sodium carboxymethylcellulose/poly(N-isopropylacrylamide)/clay semi-IPN nanocomposite hydrogels. Eur Polym J 43(6):2221–2228
Zhao L, Mitomo H (2008) Adsorption of heavy metal ions from aqueous solution onto chitosan entrapped CM-cellulose hydrogels synthesized by irradiation. J Appl Polym Sci 110:1388–1395
Duan GJ, Liu TH, Wu WS, Yang Y (2012) Adsorption of UO2 2+ from aqueous solution onto copolymers of styrene and maleic anhydride. J Radioanal Nucl Chem. doi:10.1007/s10967-012-2275-z
Zheng Y, Liu Y, Wang A (2011) Fast removal of ammonium ion using a hydrogel optimized with response surface methodology. Chem Eng J 171:1201–1208
Ansari SA, Mohapatra PK, Manchanda VK (2007) Synthesis of N,N′-dimethyl-N,N′-dibutyl malonamide functionalized polymer and its sorption affinities towards U(VI) and Th(IV) ions. Talanta 73:878–885
Pang C, Liu YH, Cao XH, Li M, Huang GL, Hua R, Wang CX, Liu YT, An XF (2011) Biosorption of uranium(VI) from aqueous solution by dead fungal biomass of Penicillium citrinum. Chem Eng J 170:1–6
Fytianos K, Voudrias E, Kokkalis E (2000) Sorption-desorption behavior of 2,4-dichlorophenol by marine sediments. Chemosphere 40:3–6
Rauf MA, Bukallah SB, Hamour FA, Nasir AS (2008) Adsorption of dyes from aqueous solutions onto sand and their kinetics behavior. Chem Eng J 137:238–243
Kay GM, Blair HS, Gardner JR (1982) Adsorption of dyes on chitin. I. Equilibrium studies. J Appl Polym Sci 27:3043–3057
Mall ID, Srivastava VC, Kumar GVA, Mishra IM (2006) Characterization and utilization of mesoporous fertilizer plant waste carbon for adsorptive removal of dyes from aqueous solution. Colloids Surf A 278:175–187
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. J1030932 and No. J51074083), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110211120038) and Fundamental Research Funds for the Central Universities (lzujbky-2012-64 and lzujbky-2012-63).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tonghuan, L., Guojian, D., Xiaojiang, D. et al. Adsorptive features of polyacrylic acid hydrogel for UO2 2+ . J Radioanal Nucl Chem 297, 119–125 (2013). https://doi.org/10.1007/s10967-012-2316-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2316-7