Abstract
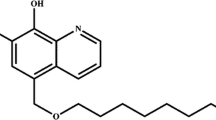
A new 8-hydroxyquinoline derivative extractant was synthesized via the Mannich reaction from a secondary amine. Various analytical techniques (1H, 13C NMR, FTIR, mass spectroscopy) were used to characterize our product. The use of this new extractant for the uptake and removal of uranyl ions in aqueous solution was investigated. Conditions for an effective sorption were optimized with respect to different experimental parameters in batch process. The results showed that the extraction rate increases for solutions with a pH in the range [0.65–1.13]. The total sorption capacity was 105 (mg g−1) under optimum experimental conditions. The extraction of UO2 2+ was found to be quantitative (100 %) at initial uranyl concentration less than or equal to 41.59 mg/L. Thermodynamic parameters showed the adsorption of an endothermic process and a spontaneous nature, respectively.








Similar content being viewed by others
References
Ludwig Hartinger (1994) Handbbok of effluent treatment and recycling for the metal finishing industry, 2nd edn. Finishing publications Ltd., Stevenage. ISBN 0-904477-14-2
Camacho LM, Deng S, Parra RR (2010) Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater 175(1–3):393–398
Phillips JP (1956) The reactions of 8-quinolinol. Chem Rev 56:271–297
Gloe K, Stephan H, Krüger T, Möckel A, Woller N, Subklew G, Schwuger MJ, Neumann R, Weber E (1996) Solvent extraction of toxic heavy metal ions with 8-hydroxyquinoline extractants from effluents. Prog Colloid Polym Sci 101:145–148
Eskew DL, Welch RM, Cary EE (1984) Effects of Ni deficiency on some nitrogen metabolites in Cowpeas (Vigna unguiculata L. walp). Plant Physiol 76:103–105
Segal I, Zablotskaya A, Lukevics E (2005) Silyl modification of biologically active compounds. Chem Heterocycl Compd 5(455):713–725
Negem NA, Morsy MIS, Said MM (2005) Biocidal activity of some Mannich base cationic derivatives. Bioorgan Med Chem 13:5921–5926
Tanzer JM, Slee AM, Kamay B, Scheer E (1978) Activity of three 8-hydroxyquinoline derivatives against in vitro dental plaque. Antimicrob Agents Chemother 13(6):1044–1045
Albrecht M, Witt K, Frôhlich R, Kataeva O (2002) Inter- and intramolecular hydrogen bonding in amide- and urea-substituted 8-hydroxyquinoline derivatives. Tetrahedron 58:561–567
García-Santos I, Sanmartín J, García-Deibe AM, Fondo M, Gómez E (2010) Structural and spectroscopic studies on some metal complexes of an 8-hydroxyquinoline derivative. Inorg Chim Acta 363(1):193–198
Himmi B, Messnaoui B, Kitane S, Eddaif A, Alaoui A, Bouklouz A, Soufiaoui M (2008) Study of Zn(II) extraction by 5-azidomethyl-8-hydroxyquinoline: experiment and modelling. Hydrometallurgy 93:39–44
Wu D, Zhang Q, Bao B (2007) Solvent extraction of Pr and Nd(III) from chloride-acetate medium by 8-hydroquinoline with and without 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester as an added synergist in heptane diluent. Hydrometallurgy 88:210–215
Sarmiento LE, Rodriguez M, Echevarria L, Lubes V (2010) Speciation of the vanadium(III) complexes with 1,10-phenanthroline, 2,2′-bipyridine, and 8-hydroxyquinoline. J Sol Chem 39:1484–1491
Salem NM, Elbraheem KAK, Mubara MS (2004) The effects of spacer groups on the chelation characteristics of some new mannich polymers containing 8-hydroxyquinoline. React Funct Polym 59:63–69
Li L, Xu B (2008) Synthesis and characterization of 5-substituted 8-hydroxyquinoline derivatives and their metal complexes. Tetrahedron 64:10986–10995
Zheng W, Miao J, Lee FSC, Xiaoru W (2006) Synthesis of 8-hydroxyquinoline bonded silica (SHQ) and its application in flow injection-inductively coupled plasma mass spectrometry analysis of trace metals in seawater. Chin J Anal Chem 34(4):459–462
Rohwer H, Rheeder N, Hosten E (1997) Interactions of uranium and thorium with arsenazoIII in an aqueous medium. Anal Chim Acta 341(2):263–268
Singh BN, Maiti B (2006) Separation and preconcentration of U(VI) on XAD-4 modified with 8-hydroxy quinoline. Talanta 69:393–396
Puigdomenech I. HYDRA (hydrochemical equilibrium-constant database) and MEDUSA (make equilibrium diagrams using sophisticated algorithms) programs. Royal Institute of Technology, Sweden. http://www.kemi.kth.se/medusa/
Kadous A, Didi MA, Villemin D (2011) Removal of uranium(VI) from acetate medium using Lewatit TP 260 resin. J Radioanal Nucl Chem 288:553–561
Kadous A, Didi MA, Villemin D (2010) A new sorbent for uranium extraction: ethylenediaminotris(methylenephosphonic) acid grafted on polystyrene resin. J Radioanal Nucl Chem 284:431–438
Filip EM, Humelnicu IV, Ghirvu CI (2009) Some aspects of 8-hydroxyquinoline in solvents. Acta Chem Iasi 17:85–96
Abderrahim O, Didi MA, Villemin D (2009) A new sorbent for uranium extraction: polyethyleniminephenylphosphonamidic acid. J Radioanal Nucl Chem 279(1):237–244
Hu H, Yang M, Dang J (2005) Treatment of strong acid dye wastewater by solvent extraction. Sep Purif Technol 42:129–136
Acknowledgments
We gratefully acknowledge the CNRS (Centre National de la Recherche Scientifique), the ‘‘Région Basse-Normandie’’ and CMEP-TASSILI N° 10 MDU799 for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zaoui, F., Didi, M.A. & Villemin, D. Investigation of 7-((dioctylamino)methyl)quinoline-8-ol for uptake and removal of uranyl ions. J Radioanal Nucl Chem 295, 419–424 (2013). https://doi.org/10.1007/s10967-012-1789-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1789-8