Abstract
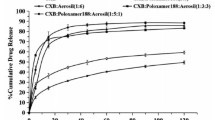
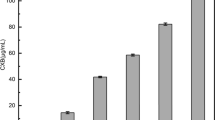
Solid dispersions can play a significant role in the enhancement of drug dissolution and stability. Still, the polymeric effect can vary according to the possibility of intermolecular forces with the drug. The objective of this study was to evaluate the effect of several polymers on enhancement in-vitro dissolution behavior of celecoxib; in addition to comparing prepared dispersions with selected commercial products. Solid dispersions of celecoxib were prepared with different ratios between the drug and selected polymer (Soluplus®, polyvinyl pyrrolidine, Chitosan, polyethylene glycol). Physicochemical characterizations were performed using Powder X-ray diffraction, Differential Scanning Calorimetry, Fourier Transform Infra-Red analysis and Scanning Electron Microscopy. Dispersions were subjected to in-vitro drug release studies. Results revealed enhancement in dissolution rate for all dispersions prepared except for Chitosan-based dispersions that showed clear retardation in the drug release. Prepared dispersions from other polymers succeeded to match with release profile of two commercially marketed products (Celebrex® and Flamex®). Further Characterization of Chitosan dispersions revealed presence celecoxib in its crystalline form entrapped inside Chitosan carrier with the presence of two hydrogen bonding between Chitosan and celecoxib. Although both Polyvinylpyrrolidone, and polyethylene glycol dispersions showed a great enhancement in drug release; both failed to maintain stability. Sticky paste formation occurred to dispersions, and recrystallization took place in polyethylene glycol dispersions.






Similar content being viewed by others
References
Rajesh K, Rajalakshmi R, Umamaheswari J, Ashok Kumar CK (2011) Liquisolid technique a novel approach to enhance solubility and bioavailability. International Journal of Biopharmaceutics 2(1):8–13
Ventura CA, Giannone I, Paolino D, Pistara V, Corsaro A, Puglisi G (2005) Preparation of celecoxib-dimethyl-beta-cyclodextrin inclusion complex: characterization and in vitro permeation study. Eur J Med Chem 40(7):624–631
Chow HHS, Anavy N, Salazar D, Frank DH, Alberts DS (2004) Determination of celecoxib in human plasma using solid-phase extraction and high-performance liquid chromatography. J Pharm Biomed Anal 34(1):167–174
Dolenc A, Kristl J, Baumgartner S, Planinsek O (2009) Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm 376(1–2):204–212
Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP (2004) Formulation Design of Self-Microemulsifying Drug Delivery Systems for improved oral bioavailability of celecoxib. Biol Pharm Bull 27(12):1993–1999
Savjani KT, Gajjar AK, Savjani JK (2012) Drug solubility: importance and enhancement techniques. International Scholarly Research Network Pharmaceutics 2012:1–10
Chokshi RJ, Zia H, Sandhu HK, Shah NH, Malick WA (2007) Improving the dissolution rate of poorly water soluble drug by solid dispersion and solid solution: pros and cons. Drug Deliv 14(1):33–45
Sekiguchi K, Obi N, Ueda Y (1964) Studies on absorption of eutectic mixture. II. Absorption of fused conglomerates of chloramphenicol and urea in rabbits. Chem Pharm Bull (Tokyo) 12:134–144
Arunachalam A, Karthikeyan M, Konam K, Prasad P, Sethuraman S, Ashutoshkumar S (2010) Solid dispersions: A review. Current Pharmaceutical Research 1(1):82–90
Breitenbach J (2002) Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm 54:107–117
Kumar BP, Rao S, Murthy KVR, Sahu RK, Ramu B (2011) Solid dispersion technique: a tool for enhancing bioavailability of poorly soluble drugs. Journal of Chemical and Pharmaceutical sciences 4(4):170–179
Vasconcelos T, Sarmento B, Costa P (2007) Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today 12(23–24):1068–1075
Obaidat RM, Tashtoush BM, Awad AA, Al Bustami RT (2016) Using supercritical fluid technology (SFT) in preparation of tacrolimus solid dispersions. AAPS PharmSciTech
Vo CL, Park C, Lee BJ (2013) Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm 85(3 Pt B):799–813
group B (2010). Soluplus Technical Information, pp 1–8
Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K (2013) Preparation of carbamazepine-Soluplus® solid dispersions by hot-melt extrusion, and prediction of drug-polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm 84(1):228–237
Thakral NK, Ray AR, Bar-Shalom D, Eriksson AH, Majumdar DK (2012) Soluplus--solubilized citrated camptothecin--a potential drug delivery strategy in colon cancer. American Association of Pharmaceutical Scientists 13(1):59–66
Fischer F, Bauer S (2009) An all rounder in the chemistry polyvinylpyrrolidone. Chem unserer Zeit 43:376–383
Folttmann H, Quadir A (2008) Polyvinylpyrrolidone (PVP) – one of the most widely used excipients in pharmaceuticals: an overview. Drug Delivery Technology 8:7–22
Shah J, Vasanti S, Anroop B, Vyas H (2008) Enhancement of dissolution rate of valdecoxib by solid dispersions technique with PVP K 30 & PEG 4000: preparation and in vitro evaluation. J Incl Phenom Macrocycl Chem 63(1–2):69–75
Singh A, Sharma PK, Meher JG, Malviya R (2011) Evaluation of enhancement of solubility of paracetamol by solid dispersion technique using different polymers concentration. Asian Journal of Pharmaceutical and Clinical Research 4(1):117–119
Badawy MEI, Rabea EI (2011) A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. International Journal of Carbohydrate Chemistry 2011:1–29
de Alvarenga ES (2011) Characterization and properties of chitosan. In: Biotechnology of biopolymers, pp 91–108
Rodrigues S, Dionisio M, Lopez CR, Grenha A (2012) Biocompatibility of chitosan carriers with application in drug delivery. Journal of Functional Biomaterials 3(3):615–641
Caliceti P (2003) Pharmacokinetic and biodistribution properties of poly(ethylene glycol)–protein conjugates. Adv Drug Deliv Rev 55(10):1261–1277
Kumar SGV, Mishra DM (2006) Preparation, characterization and in vitro dissolution studies of solid dispersion of meloxicam with PEG 6000. The Pharmaceutical Society of Japan 126(8):567–664
Obaidat R, Al-Jbour N, Al-Sou’d K, Sweidan K, Al-Remawi M, Badwan A (2010) (2010). Some Physico-chemical properties of low molecular weight Chitosans and their relationship to conformation in aqueous solution. J Solut Chem 39(4):575–588
Obaidat RM, Tashtoush BM, Bayan MF, Al Bustami RT, Mohammad A (2015) Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. American Association of Pharmaceutical Scientists. 16(6):1235–1244
Primo FT, Froehlich PE (2005) Celecoxib identification methods. Acta Farm Bonaer 24(3):421–425
Schott H, Kwan LC, Feldman S (1982) The role of surfactants in the release of very slightly soluble drugs from tablets. J Pharm Sci 71(9):1038–1045
Chawdary KPR, Srinivas SV (2006) Influence of hydrophilic polymers on CelecoxibComplexation with Hydroxypropyl β-cyclodextrin. American Association of Pharmaceutical Scientists. 7(3):E1–E6
Nasr M (2010) In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. American Association of Pharmaceutical Scientists 11(1):85–89
Shoaib MH, Tazeen J, Merchant HA, Yousuf RI (2006) Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci 19(2):119–124
Parize AL, Stulzer HK (2012) Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray dryin. Quim Nov. 35(6):1127–1132
Jadhav K, Gowekar N, Gowekar S (2012) A validated RP-HPLC method for the determination of celecoxib in bulk and pharmaceutical dosage form. Int J Res Pharmaceut Biomed Sci 3(3):1312–1316
Lee JH, Kim MJ, Yoon H, Shim CR, Ko HA, Cho SA, et al (2013) Enhanced dissolution rate of celecoxib using PVP and/or HPMC-based solid dispersions prepared by spray drying method. Journal of Pharmaceutical Investigation 43(3):205–213
Caron V, Hu Y, Tajber L, Erxleben A, Corrigan OI, McArdle P, et al (2013) Amorphous solid dispersions of sulfonamide/Soluplus(R) and sulfonamide/PVP prepared by ball milling. American Association of Pharmaceutical Scientists 14(1):464–474
Chawla G, Gupta P, Thilagavathi R, Chakraborti AK, Bansal AK (2003) Characterization of solid-state forms of celecoxib. Eur J Pharm Sci 20(3):305–317
Gupta VR, Mutalik S, Patel MM, Jani GK (2007) Spherical crystals of celecoxib to improve solubility, dissolution rate and micromeritic properties. Acta Pharma 57:173–184
Shamma RN, Basha M (2013) Soluplus®: a novel polymeric solubilizer for optimization of carvedilol solid dispersions: formulation design and effect of method of preparation. Powder Technol 237:406–414
Sethia S, Squillante E (2004) Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm 272(1–2):1–10
Ujang Z, Diah M, Abdul Rashid AH, Halim AS (2011) The development, characterization and application of water soluble chitosan. Biotechnol Biopolymers 109–31
El-Badry M, Fetih G, Fathy M (2009) Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharmaceutical Journal 17(3):217–225
Patil M, Gaikwad N (2011) Characterization of gliclazide-polyethylene glycol solid dispersion and its effect on dissolution. Brazilian Journal of Pharmaceutical Sciences 47:161–166
Homayouni A, Sadeghi F, Nokhodchi A, Varshosaz J, Garekani HA, Vyas H (2014) Preparation and characterization of celecoxib solid dispersions; comparison of poloxamer-188 and PVP-K30 as carriers. Iranian Journal of Basic Medical Sciences 17:322–331
Homayouni A, Sadeghi F, Nokhodchi A, Varshosaz J, Garekani HF (2015) Preparation and characterization of celecoxib dispersions in Soluplus®: comparison of spray drying and conventional methods. Iranian Journal of Pharmaceutical Research 14(1):35–50
Zhang X, Sun N, Wu B, Lu Y, Guan T, Wu W (2008) Physical characterization of lansoprazole/PVP solid dispersion prepared by fluid-bed coating technique. Powder Technol 182(3):480–485
Guleria R, Kaith NS, Singh R (2012) PEG based solid dispersions of gliclazide a comparative study. Int J Pharm Pharm Sci 4(1):507–511
Sambhakar S, Singh B, Madan K, Singh M, Kashyap N, Mayle S (2013) Solid dispersions: a tool for improving the solubility and dissolution of metronidazole. International Journal of Drug Delivery 5:94–98
Hu L, Gu D, Hu Q, Shi Y, Gao N (2014) Investigation of solid dispersion of atorvastatin calcium in polyethylene glycol 6000 and polyvinylpyrrolidone. Trop J Pharm Res 13(6):835
Liu C, Desai KG, Liu C (2005) Enhancement of dissolution rate of valdecoxib using solid dispersions with polyethylene glycol 4000. Drug Dev Ind Pharm 31(1):1–10
Fouad EA, El-Badry M, Mahrous GM, Alanazi FK, Neau SH, Alsarra IA (2011) The use of spray-drying to enhance celecoxib solubility. Drug Dev Ind Pharm 37(12):1463–1472
Naelapää K, Boetker J, Müllertz A, Rades T, Rantanen J, Bar-Shalom D. Soluplus® for modifying the release of highly water soluble APIs
Tantishaiyakul V, Kaewnopparat N, Ingkatawornwong S (1996) Properties of solid dispersions of piroxicam in polyvinylpyrrolidone K-30. Int J Pharm 143:59–66
Verheyen S, Blaton N, Kinget R, Van den Mooter G (2002) Mechanism of increased dissolution of diazepam and temazepam from polyethylene glycol 6000 solid dispersions. Int J Pharm 249:45–58
Dangprasirt P, Ritthidej G (1995) Development of diclofenac sodium controlled release solid dispersions by spray drying using optimization strategy I. Powder formulation. Drug Dev Ind Pharm 21(20):2323–2337
Soliman M, Abdel Malak NS, El Gazayerly ON, Abdel Rehim AA (2011) Preparation of celecoxib solid dispersions for dermal application: in vitro characterization and skin irritation test. J Drug Del Sci Tech 21(6):509–516
Kim EJ, Chun MK, Jang JS, Lee IH, Lee KR, Choi HK (2006) Preparation of a solid dispersion of felodipine using a solvent wetting method. Eur J Pharm Biopharm 64(2):200–205
Sekikawa H, Fujiwara JE, Naganuma T, Nakano M, Arita T (1987) Inhibitory effect of polyvinylpyrrolidone on the crystallization of drugs. Chem Pharm Bull (Tokyo) 26:118–126
Leuner C, Dressman J (2000) Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 50:47–60
Craig DQM (2002) The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 231:131–144
Katharya AK, Chaudhary R, Sharma R, Singh Y, Teotia UVS (2013) Development and optimization of solid dispersion of olanzapine in poly ethylene glycol by D-optimal response surface factorial design. International Journal of PharmTech Research 5(2):700–710
Narang AS, Srivastava AK (2002) Evaluation of solid dispersions of Clofazimine. Drug Dev Ind Pharm 28(8):1001–1013
Ullah M, Ullah H, Murtaza G, Mahmood Q, Hussain I (2015) Evaluation of influence of various polymers on dissolution and phase behavior of carbamazepine-succinic acid Cocrystal in matrix tablets. Biomed Res Int 2015:1–10
Abd Alaziz DM, Sammour OA, Elshamy AEAA, Neseem DI (2014) Formulation and evaluation of binary and ternary solid dispersions of domperidone by solvent evaporation method. Afr J Pharm Pharmacol 8(3):66–80
Parmar KR, Shah SR, Sheth NR (2011) Studies in dissolution enhancement of Ezetimibe by solid dispersions in combination with a surface adsorbent. Dissolution Technologies 18(3):55–61
Afifia S (2015) Solid dispersion approach improving dissolution rate of Stiripentol: a novel antiepileptic drug. Iranian Journal of Pharmaceutical Research 14(4):1001–1014
Ahuja N, Katare OP, Singh B (2007) Studies on dissolution enhancement and mathematical modeling of drug release of a poorly water-soluble drug using water-soluble carriers. Eur J Pharm Biopharm 65(1):26–38
Murtaza G, Ahmad M, Khan S, Hussain I (2012) Evaluation of Cefixime-loaded chitosan microspheres: analysis of dissolution data using DDSolver. Dissolution Technologies 19(2):13–19
Raghavendra Rao NG, Kulkarni U, Deshmukh A, Suresh DK (2010) Preparation and characterization of Ionotropic cross-linked chitosan microparticles for controlled release of Aceclofenac. International Journal of Pharmaceutical Sciences and Drug Research 2(2):107–111
Acknowledgements
This work is funded by Deanship of Research at Jordan University Science and Technology (JUST) with Fund number (302/2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obaidat, R.M., AlTaani, B. & Ailabouni, A. Effect of different polymeric dispersions on In-vitro dissolution rate and stability of celecoxib class II drug. J Polym Res 24, 58 (2017). https://doi.org/10.1007/s10965-017-1215-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1215-6