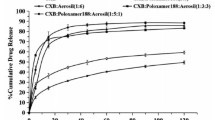
Celecoxib, the first cyclooxygenase-2 selective nonsteroidal anti-inflammatory drug, has a wide range of pharmacological effects, including anti-inflammatory, antirheumatic and antitumor effects. However, celecoxib’s poor solubility severely limits its clinical application. Thus, a novel ternary solid dispersion system was developed using PVP K30 and hydroxypropyl-β-cyclodextrin (HPβCD) as carrier materials in order to improve its solubility and dissolution behavior. The solid dispersion system was characterized by SEM, XRD, FT-IR, DSC, in vitro dissolution, saturated solubility, and contact angle test; the stability of the samples under extreme environments was investigated as well. The results showed that the novel ternary solid dispersion doped with PVP K30 and HPβCD has enhanced solubility around 6.28-fold, an almost 13.94-fold increase in the dissolution rate (120 min) compared with celecoxib’s active pharmaceutical ingredient. The substantial improvement in its dissolution performance is attributed to the fact that celecoxib is dispersed in the carrier material in an amorphous form and has hydrogen bonding interactions with the carrier materials. In addition, the improvement of wettability is one of the reasons for its increased solubility. In conclusion, the combination of PVP K30 and HPβCD can significantly improve the solubility of celecoxib and this novel solid dispersion system provides new ideas and methods to further improve the solubility of insoluble drugs.










Similar content being viewed by others
References
N. M. Davies, A. J. McLachlan, R. O. Day, et al., Clin. Pharmacokinet., 38, 225 – 242 (2000).
J. Li, Q. Hao, W. Cao, et al., Cancer Manage. Res., 10, 4653 – 4667 (2018).
N. Tołoczko-Iwaniuk, D. Dziemiańczyk-Pakieła, K. B. Nowaszewska, et al., Curr. Drug Targets, 20, 302 – 315 (2019).
K. S. Ahmed, S. Changling, X. Shan, et al., J. Liposome Res., 30, 285 – 296 (2020).
A. H. Schönthal, T. C. Chen, F. M. Hofman, et al., Expert Opin. Invest. Drugs., 17, 197 – 208 (2008).
Þ. G. Küçükgüzel, Ý. Coþkun, S. Aydýn, et al., Molecules., 18, 3595 – 3614 (2013).
D. Clemett, K. L. Goa, Drugs, 59, 957 – 980 (2000).
F. Norouzi, A. Jouyban, F. Martinez, et al., Phys. Chem. Liq., 57, 755 – 767 (2019).
X. He, M. R. Barone, P. J. Marsac, et al., Int. J. Pharm., 353, 176 – 186 (2008).
C. Leuner, J. Dressman, Eur. J. Pharm. Biopharm., 50, 47 – 60 (2000).
T. Turovsky, R. Khalfin, S. Kababya, et al., Langmuir, 31, 7183 – 7192 (2015).
H.-I. Kim, S. Y. Park, S. J. Park, et al., Pharmaceutics, 10 (2018).
I. Hwang, V. Renuka, J.-H. Lee, et al., Pharm. Dev. Technol., 25, 525 – 534 (2020).
S. Mukesh, P. Joshi, A. K. Bansal, et al., Mol. Pharm., 18, 2334 – 2348 (2021).
T. Vasconcelos, S. Marques, J. das Neves, et al., Adv. Drug Delivery Rev., 100, 85 – 101 (2016).
T. Xie, L. S. Taylor, Pharm. Res., 33, 739 – 750 (2016).
M. Maghsoodi, A. Nokhodchi, H. Pourasghari Azar, Pharm. Dev. Technol., 26, 788 – 796 (2021).
K. R Berziņš, S. J. Fraser-Miller, G. F. Walker, et al., Mol. Pharm., 18, 3882 – 3893 (2021).
D. E. Moseson, I. D. Corum, A. Lust, et al., The AAPS Journal, 23, 69 (2021).
E.-J. Heo, S. Y. Park, H.-I. Kim, et al., J. Nanosci. Nanotechnol., 20, 5813 – 5818 (2020).
A. Niederquell, E. Stoyanov, M. Kuentz, Mol. Pharm., 19, 690 – 703 (2022).
H. J. Kwon, E.-J. Heo, Y.-H. Kim, et al., Pharmaceutics, 11(3), 136 (2019).
C. M. De Jongh, M. M. Verberk, C. E. T. Withagen, et al., Contact Dermatitis, 54, 325 – 333 (2006).
K. H. Park, J. M. Choi, E. Cho, et al., Polymers, 10, 111 (2018).
X. Yang, J. Shen, J. Liu, et al., Crystals, 12, 596 (2022).
A. Homayouni, F. Sadeghi, A. Nokhodchi, et al., Iran J. Basic Med. Sci., 17, 322 – 331 (2014).
R. Ghanavati, A. Taheri, A. Homayouni, Materials Science and Engineering: C, 72, 501 – 511 (2017).
A. Zoghbi, T. Geng, B. Wang, AAPS PharmSciTech, 18, 2927 – 2935 (2017).
K. O. Boakye-Yiadom, S. Kesse, M. Aquib, et al., Polym. Adv. Technol., 31, 2270 – 2278 (2020).
V. R. Sinha, R. Anitha, S. Ghosh, et al., Acta Pharmaceutica (Zagreb, Croatia), 57, 47 – 60 (2007).
C. M. Hsu, S. C. Yu, F. J. Tsai, et al., Colloids and Surfaces. B. Biointerfaces, 180, 68 – 74 (2019).
A. Homayouni, F. Sadeghi, A. Nokhodchi, et al., Iran J. Basic Med. Sci., 17, 322 – 331 (2014).
J. A. Baird, L. S. Taylor, Adv. Drug Deliv. Rev., 64, 396 – 421 (2012).
S. Verma, V. S. Rudraraju, AAPS PharmSciTech, 16, 1079 – 1090 (2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Ouyang, F., Li, T. et al. Ternary Solid Dispersion of Celecoxib Produced by the Solvent Method with Improved Solubility and Dissolution Properties. Pharm Chem J 57, 1627–1636 (2024). https://doi.org/10.1007/s11094-024-03058-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03058-5