Abstract
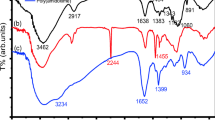
A new adsorbent (AM-Fe-PGCP), iron(III) complex of an amino-functionalized poly(acrylamide)-grafted coconut coir pith(CP) was prepared through graft copolymerization of acrylamide onto CP (a lignocellulosic residue) in the presence of N, N′-methylenebisacrylamide as cross-linker using and potassium persulphate as an initiator, followed by loading with Fe(III) in the presence of HCl and was tested for its ability to recover chromium(VI) from water and industry effluents. The adsorbent was characterized using FTIR, SEM, XRD, TG/DTG, Surface area analyzer and potentiometric titrations. The potential of the AM-Fe-PGCP to adsorb Cr(VI) from aqueous solutions was investigated under different optimized conditions of pH, concentration of Cr(VI), contact time, and temperature. The effective pH for the removal of Cr(VI) was 4.0. Kinetic data followed a pseudo-second-order model. The equilibrium data were correlated with the Langmuir isotherm model. The equilibrium Cr(VI) sorption capacity was estimated to be 90.09 mg g-1. Quantitative removal of 27.7 mg L-1 Cr(VI) from 1.0 L of electroplating industry wastewater was achieved by 0.5 g adsorbent. The reusability of the adsorbent was demonstrated over four cycles using 0.1 M NaOH solution.










Similar content being viewed by others
References
Chiarle S, Ratto M, Rovatti M (2000) Water Res 34:2971
Environmental Protection Agency (EPA). http://www.epa.gov
Gardea-Torresday JR, de la Rosa G, Peralta-Videa JR (2004) Pure Appl Chem 76:801
Anirudhan TS, Unnithan MR (2007) Chemosphere 66:60
Babel S, Kurniawan TA (2003) J Hazard Mater B97:219
Saliba R, Gauthier H, Guathier R, Petit-Ramel M (2000) J Appl Polym Sci 75:1624
Mohan D, Pittman CU (2007) J Hazard Mater 142:1–53
Amin MN, Kaneco KT, Begum A, Katsumata H, Suzuki T, Ohta K (2006) Ind Eng Chem Res 45:8105
Shibi IG, Anirudhan TS (2006) J Chem Technol Biotechnol 81:433
Noelin BF, Manohar DM, Anirudhan TS (2005) Sep Purif Technol 45:131
Gopal M, Gupta RA (2000) Indian Coconut J 31:13
Ott E (1946) Cellulose and cellulose derivatives. Interscience, NewYork
Greenberg AE, Clescerl LS, Eaton AD (1992) Standard Method for the Examination of Water and Wastewater, 18th ed.; APHA, AWWA, and WEF. Washington. D.C.
Schwarz JA, Driscoll CT, Bhanot AK (1984) J Colloid Interface Sci 97:55
Kotaś J, Stasicka Z (2000) Environ Poll 107(3):263
Benefield LD, Judkins JP, Wend BL (1982) Process chemistry for water and wastewater treatment, Prentice Hall, Englewood Cliffs, NJ, activated charcoal. Chem Zentr 1:875
Anirudhan TS, Suchithra PS (2007) Ind Eng Chem Res 46:4606
Marquardt DW (1963) J Soc Ind Appl Math 11:431
Cimino G, Passerini A (2000) Water Res 34:2955
Brito F, Ascanio J, Mateo S, Hernandez C, Araujo L, Gili P, Martin-Zarza P, Dominguez S, Mederos A (1997) Polyhedron 16:3835
Seader JD, Henley EJ (eds) (1998) Separation process principles. Wiley, New York
Freundlich HMF (1906) Z Phys Chem 57:385
Hall KR, Engleton LC, Acrivas A, Vermeulen T (1966) Ind Eng Chem Fund 5:212
Sharma DC, Forster CF (1995) Process Biochem 30:293
Young DM, Crowell AD (1962) Physical adsorption of gases. Butterworth, London
Maya R, Vinod VP, Anirudhan TS (2004) Ind Eng Chem Res 43:2247
Sharma DC, Foster CF (1994) Bioresource Technol 47:257
Sarin V, Pant KK (2006) Bioresource Technol 97:15
Schmuhl R, Krieg HM, Keizer K (2001) Water SA 27:1
Tang PL, Lee CK, Low KS, Zainal Z (2003) Environ Technol 24:1243
Acknowledgements
The authors are thankful to the Head, Department of Chemistry, University of Kerala, Trivandrum for providing laboratory facilities and Mr. S. Rijith express his sincere thanks to the University Grant Commission, New Delhi for the financial support in the form of Research Fellowship to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anirudhan, T.S., Rijith, S. & Das Bringle, C. Iron(III) complex of an amino-functionalized poly(acrylamide)-grafted lignocellulosic residue as a potential adsorbent for the removal of chromium(VI) from water and industry effluents. J Polym Res 17, 289–299 (2010). https://doi.org/10.1007/s10965-009-9316-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-009-9316-5