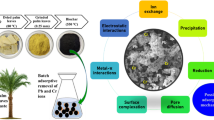
Abstract
A waste material known as palm oil empty fruit bunch (EFB) is used as a source of cellulose for the development of polymeric materials for the removal of metal ions from industrial wastewater. A poly(acrylonitrile)-grafted palm cellulose copolymer was synthesized by a conventional free radical initiating process followed by synthesis of a poly(amidoxime) ligand by oximation reaction. The resulting products were characterized by FT-IR, FE-SEM, EDX, TGA, DSC, and XPS. The poly(amidoxime) ligand was used to coordinate with and extract a series of transition metal ions from water samples. The binding capacity (qe) of the ligand with the metal ions such as copper, iron, cobalt, nickel, and lead were 260, 210, 168, 172, and 272 mg g−1, respectively at pH 6. The adsorption process followed the pseudo-first-order kinetic model (R2 > 0.99) and as well as the Freundlich isotherm model (R2 > 0.99) indicating the occurrence of a multi-layer adsorption process in the amidoxime ligand adsorbent. Results from reusability studies show that the ligand can be recycled for at least 10 cycles without any significant losses to its initial adsorption capacity. The synthesized polymeric ligand was shown to absorb heavy metals from electroplating wastewater with up to 95% efficiency.














Similar content being viewed by others
References
Ahmad M, Ahmed S, Swami BL, Ikram S (2015) Adsorption of heavy metal ions: role of chitosan and cellulose for water treatment. Langmuir 79:109–155
Ahmadpour A, Eftekhari N, Ayati A (2014) Performance of MWCNTs and a low-cost adsorbent for chromium (VI) ion removal. J Nanostruct Chem 4(4):171–178
Anirudhan T, Nima J, Divya P (2013) Adsorption of chromium (VI) from aqueous solutions by glycidylmethacrylate-grafted-densified cellulose with quaternary ammonium groups. Appl Surf Sci 279:441–449
Arif N, Yadav V, Singh S, Singh S, Ahmad P, Mishra RK, Sharma S, Tripathi DK, Dubey NK, Chauhan DK (2016) Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front Environ Sci 4:1–11
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017
Beccia MR, García B, García-Tojal J, Leal JM, Secco F, Tegoni M (2014) The mechanism of the Cu 2+[12-MC Cu (Alaha)-4] metallacrown formation and lanthanum (iii) encapsulation. Dalton Trans 43(24):9271–9282
Bopda A, Tchuifon TDR, Nche GNA, Kamdem TA, Anagho SG (2018) Adsorption of 2,4-dinitrophenol on activated carbon prepared from cotton cakes: non linear isotherm modelling. Chem Sci Int J 24(1):1–20
Chen X, Liu L, Luo Z, Shen J, Ni Q, Yao J (2018) Facile preparation of a cellulose-based bioadsorbent modified by hPEI in heterogeneous system for high-efficiency removal of multiple types of dyes. React Funct Polym 125:77–83
Donia AM, Yousif AM, Atia AA, Abd El-Latif HM (2014) Preparation and characterization of modified cellulose adsorbents with high surface area and high adsorption affinity for Hg (II). J Disper Sci Technol 35(3):380–389
dos Santos Silva L, de Oliveira Carvalho J, de Sousa Bezerra RD, da Silva M, Ferreira F, Osajima J, da Silva Filho E (2018) Potential of cellulose functionalized with carboxylic acid as biosorbent for the removal of cationic dyes in aqueous solution. Molecules 23(4):743
Dwivedi AD, Dubey SP, Hokkanen S, Fallah RN, Sillanpää (2014) Recovery of gold from aqueous solutions by taurine modified cellulose: an adsorptive–reduction pathway. Chem Eng J 255:97–106
Eigen M, Tamm K (1962) Sound absorption in electrolytes as a consequence of chemical relaxation. I. Relaxation theory of stepwise dissociation. Z. Elektrochem 66:107–121
Eigen M, Wilkins RG (1965) The kinetics and mechanism of formation of metal complexes. ACS Publications, Washington, D.C.
Fu J, Zhao C, Luo Y, Liu C, Kyzas GZ, Luo Y, Zhao D, An S, Zhu H (2014) Heavy metals in surface sediments of the Jialu River, China: their relations to environmental factors. J Hazard Mater 270:102–109
Furushima Y, Nakada M, Takahashi H, Ishikiriyama K (2014) Study of melting and crystallization behavior of polyacrylonitrile using ultrafast differential scanning calorimetry. Polymer 55(13):3075–3081
Hajeeth T, Vijayalakshmi K, Gomathi T, Sudha P (2013) Removal of Cu (II) and Ni (II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int J Biol Macromol 62:59–65
Helm L, Merbach A (1999) Water exchange on metal ions: experiments and simulations. Coord Chem Rev 187(1):151–181
Hokkanen S, Bhatnagar A, Sillanpää M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173
Hong QL, Yong SQ, Jian X (2015) Microwave-assisted conversion of lignin. Biofuels Biorefineries 3:61–82
Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, Pugazhendhi A (2018) Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manag 217:56–70
Jang HM, Yoo S, Choi YK, Park S, Kan E (2018) Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour Technol 259:24–31
Ju A, Guang S, Xu H (2013) Effect of comonomer structure on the stabilization and spinnability of polyacrylonitrile copolymers. Carbon 54:323–335
Kiani GR, Sheikhloie H, Arsalani N (2011) Heavy metal ion removal from aqueous solutions by functionalized polyacrylonitrile. Desalination 269:266–270
Kubota H, Shigehisa Y (1995) Introduction of amidoxime groups into cellulose and its ability to adsorb metal ions. J Appl Polym Sci 56(2):147–151
Lin G, Wang S, Zhang L, Hu T, Peng J, Cheng S, Fu L, Srinivasakannan C (2018) Selective recovery of Au (III) from aqueous solutions using 2-aminothiazole functionalized corn bract as low-cost bioadsorbent. J Clea Prod 196:1007–1015
Liu L, Xie JP, Li YJ, Zhang Q, Yao JM (2016) Three-dimensional macroporous cellulose-based bioadsorbents for efficient removal of nickel ions from aqueous solution. Cellulose 23(1):723–736
Long H, Zhao Z, Chai Y, Li X, Hua Z, Xiao Y, Yang Y (2015) Binding mechanism of the amidoxime functional group on chelating resins toward gallium (III) in Bayer liquor. Ind Eng Chem Res 54(33):8025–8030
Lutfor MR, Silong S, Md Zin W, Ab. Rahman MZ, Ahmad M, Haron J (2000) Preparation and characterization of poly(amidoxime) chelating resin from polyacrylonitrile grafted sago starch. Eur Polymer J 36(10):2105–2113
O’Connell DW, Birkinshaw C, O’Dwyer TF (2006a) A modified cellulose adsorbent for the removal of nickel (II) from aqueous solutions. J Chem Technol Biotechnol 81(11):1820–1828
O’Connell D, Birkinshaw C, O’Dwyer T (2006b) A chelating cellulose adsorbent for the removal of Cu (II) from aqueous solutions. J Appl Polym Sci 99(6):2888–2897
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour Technol 99(15):6709–6724
Pan Y, Wang F, Wei T, Zhang C, Xiao H (2016) Hydrophobic modification of bagasse cellulose fibers with cationic latex: adsorption kinetics and mechanism. Chem Eng J 302:33–43
Pan Y, Shi X, Cai P, Guo T, Tong Z, Xiao H (2018) Dye removal from single and binary systems using gel-like bioadsorbent based on functional-modified cellulose. Cellulose 25(4):2559–2575
Rahman ML, Sarkar SM, Yusoff MM, Abdullah MH (2016a) Efficient removal of transition metal ions using poly (amidoxime) ligand from polymer grafted kenaf cellulose. RSC Adv 6(1):745–757
Rahman ML, Sarkar SM, Yusoff MM, Kulkarni AKD, Chowdhury ZZ, Ali ME (2016b) Poly(amidoxime) from polymer-grafted khaya cellulose: an excellent medium for the removal of transition metal cations from aqueous solution. Bioresources 11(3):6780–6800
Rahman ML, Sarkar SM, Yusoff MM, Abdullah MH (2017) Optical detection and efficient removal of transition metal ions from water using poly (hydroxamic acid) ligand. Sensor Actuat B Chem 242:595–608
Robson FR, Luiz CP, Nilton PA, Carlos ARBJ (2014) Synthesis and thermal behavior of polyacrylonitrile/vinylidene chloride copolymer. Polimeros 24(3):259–268
Robson FR, Luiz CP, Nilton PA, Carlos ARBJ (2015) Thermal stabilization study of polyacrylonitrile fiber obtained by extrusion. Polimeros 25(6):523–530
Salah M, El-Bahy ZM, El-Bahy (2015) Synthesis and characterization of polyamidoxime chelating resin for adsorption of Cu (II), Mn(II) and Ni(II) by batch and column study. J Envriron Chen Eng 4(1):276–286
Sanchooli MM, Rahdar S, Tanghavi M (2016) Cadmium removal from aqueous solutions using saxaul tree ash. Iran J Chem Eng 35(3):45–52
Silva SM, Sampaio KA, Ceriani R, Verhé R, Stevens C, De Greyt W, Meirelles AJ (2013) Adsorption of carotenes and phosphorus from palm oil onto acid activated bleaching earth: equilibrium, kinetics and thermodynamics. J Food Eng 118(4):341–349
Wani AL, Anjum A, Jawed AU (2015) Lead toxicity: a review. Interdiscip Toxicol 8(2):55–64
Wilkins R (1964) The kinetics of formation of some divalent transition metal-dye complexes, studied by the temperature-jump relaxation method. Inorg Chem 3(4):520–522
Wu W, Wu P, Yang F, Sun DI, Zhang DX, Zhou YK (2018) Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci Total Environ 630:53–61
Yousef RI, El-Eswed B, Ala’a H (2011) Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: kinetics, mechanism, and thermodynamics studies. Chem Eng J 171(3):1143–1149
Zheng L, Dang Z, Yi X, Zhang H (2010) Equilibrium and kinetic studies of adsorption of Cd (II) from aqueous solution using modified corn stalk. J Hazard Mater 176(1–3):650–656
Associated content
The experimental part containing detailed preparation procedure and characterization is described.
Funding
This research work was supported by the Universiti Malaysia Sabah (SGI0061-2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, M.L., Fui, C.J., Sarjadi, M.S. et al. Poly(amidoxime) ligand derived from waste palm fiber for the removal of heavy metals from electroplating wastewater. Environ Sci Pollut Res 27, 34541–34556 (2020). https://doi.org/10.1007/s11356-020-09462-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09462-0