Abstract
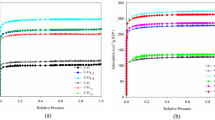
The kinetics and thermodynamics of water adsorption onto rice husks ash filled polypropene composites during soaking were studied at different temperatures, quantities and nature of fillers. Raw rice husk, “white” and “black” rice husks ash and Aerosil were used as fillers of polypropene. The increase of fillers contents in the polymer matrix was found to result in non-linear increase of the amount of adsorbed water. The highest adsorption capacity showed the samples filled with raw rice husks, while the lowest—those filled with black rice husks ash. The adsorption kinetics was limited by intra-particle diffusion in plane sheet particles. The values of the diffusion coefficients D, diffusion constants D o, activation energy of the diffusion process Е а, changes of free energy ΔG ≠, enthalpy ΔH ≠ and entropy ΔS ≠ for the formation of the activated complex from the reagent were calculated. A compensation effect between D o and Е а was observed. Based on the Van’t Hoff equation, the values of the changes of standard free energy ΔG o, enthalpy ΔH o and entropy ΔS o of water adsorption were calculated. The sorption process is exothermal in nature and accompanied with decrease of the entropy. The values of the sorption coefficient S and permeability coefficient P were calculated at 25 and 90 °C.













Similar content being viewed by others
Abbreviations
- PP:
-
Polypropene
- RRH:
-
Raw rice husks
- WRHA:
-
White rice husks ash
- BRHA:
-
Black rice husks ash
- AR:
-
Aerosil A200, Degussa
- B 1 :
-
The slope of the plot \(F = {\text{f}}\left( {\sqrt t } \right)\)
- B 2 :
-
The slope of the plot −ln(1 − F) = f(t)
- C :
-
The concentration of fillers (mass.%)
- D :
-
The diffusion coefficient (m2 s−1)
- D o :
-
The diffusion constant (m2 s−1)
- D iso :
-
The iso-kinetics diffusion coefficient (m2 s−1)
- E a :
-
The activation energy of the sorption process (kJ mol−1)
- F :
-
The fraction of sorbed water for period of times t (dimensionless)
- K e :
-
The equilibrium constant
- L :
-
The thickness of the slab (cm)
- Q t :
-
The water uptake at time t (g)
- \(Q_\infty \) :
-
The water uptake at equilibrium (g)
- P :
-
The permeability coefficient (m2 s−1)
- R :
-
The universal gas constant (8.314 J mol−1K−1)
- S :
-
The sorption coefficient (g g−1)
- T :
-
The absolute temperature (K)
- T iso :
-
The iso-kinetics temperature (K)
- W o :
-
The initial weight of the dry sample (g)
- W e :
-
The weight of the wet sample at equilibrium at a given temperature (g)
- K e :
-
The equilibrium constant
- ΔG o :
-
The change of standard free energy of absorption (kJ mol−1)
- ΔH o :
-
The change of standard enthalpy of absorption (kJ mol−1)
- ΔS o :
-
The change of standard entropy of absorption (J mol−1K−1)
- ΔG ≠ :
-
The change of free energy for the formation of the activated complex from the reagent (kJ mol−1)
- ΔH ≠ :
-
The change of enthalpy for the formation of the activated complex from the reagent (kJ mol−1)
- ΔS ≠ :
-
The change of entropy for the formation of the activated complex from the reagent (J mol−1K−1)
- d :
-
The average molecular jump distance assuming equal to 5 × 10−10 m
- e :
-
The Neper number, equal to 2.7183
- h :
-
The Planck’s constant (6.626 × 10−34 J s)
- k B :
-
The Boltzmann constant (1.38 × 10−23 J mol−1K−1)
- m e :
-
The mass of the water taken up at equilibrium (g)
- r :
-
The radius of cylinder or fiber (cm)
- r o :
-
The radius of the spherical particles (cm)
- t :
-
Time (h)
- α :
-
The rate of chemisorption at zero coverage or the initial adsorption rate (mg g−1 min−1)
- α n :
-
The roots of the first species of Bessel’s function and order 0 (dimensionless)
- β :
-
The extent of surface coverage and the activation energy involved in chemisorption (g mg−1)
References
Ishak ZAM, Yow BN, Ng BL, Khalil HPSA, Rozman HD (2001) J Appl Polym Sci 81:742 doi:10.1002/app.1491
Premalal HGB, Ismail H, Baharin A (2003) Polym Plast Technol Eng 42:827 doi:10.1081/PPT-120024998
Ismail H, Mohamad Z, Bakar AA (2003) Polym Plast Technol Eng 42:81 doi:10.1081/PPT-120016337
Gouanve E, Marais S, Bessadok A, Langevin D, Metayer M (2007) Eur Polym J 43:586 doi:10.1016/j.eurpolymj.2006.10.023
Pehlivan H, Ozmihci F, Tihminlioglu T, Ulku S (2003) J Appl Polym Sci 90:3069 doi:10.1002/app.13046
Joly C, Gauthier R, Escoubes M (1996) J Appl Polym Sci 61:57 doi:10.1002/(SICI)1097-4628(19960705)61:1<57::AID-APP7>3.0.CO;2-T
Jacob M, Varughese KT, Thomas S (2005) Biomacromolecules 6:2969 doi:10.1021/bm050278p
Razavi-Nouri M, Dogouri FJ, Oromiehie A, Langroudi AE (2006) Iranian. Polym J 15:757
Kumar U, Bandyopadhyay M (2006) Bioresour Technol 97:104 doi:10.1016/j.biortech.2005.02.027
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) Water Res 33:2469 doi:10.1016/S0043-1354(98)00475-8
Chuah TG, Jumasiah A, Aznu I, Katayon S, Thomas Choong SY (2005) Desalination 175:305 doi:10.1016/j.desal.2004.10.014
Fuad MYA, Ismail Z, Mansor MS, Ishak ZAM, Omar AKM (1995) Polym J 27:1002 doi:10.1295/polymj.27.1002
Chaudhary DS, Jollands MC, Cser F (2002) Silicon Chem 1:281 doi:10.1023/B:SILC.0000018361.66866.80
Premalal HGB, Ismail H, Baharin A (2002) Polym Test 21:833 doi:10.1016/S0142-9418(02)00018-1
Panthapulakkal S, Law S, Sain M (2005) J Thermoplastic Comp Mater 18:445 doi:10.1177/0892705705054398
Toro P, Quijada R, Murillo O, Yazdani-Pedram M (2005) Polym Int 54:30 doi:10.1002/pi.1740
Misra S, Verma J (2006) J Appl Polym Sci 101:2530 doi:10.1002/app.23916
Namasivayam C, Sangeetha D, Gunasekharan R (2007) Process Saf Environ Prot 85(B2):181 doi:10.1205/psep05002
Boyd GE, Adamson AW, Myers LS Jr (1947) J Am Chem Soc 69:2836 doi:10.1021/ja01203a066
Noroozi B, Sorial GA, Bahrami H, Arami M (2007) J Hazard Mater B139:167 doi:10.1016/j.jhazmat.2006.06.021
Ton-That TM, Jungnickel B-J (1999) J Appl Polym Sci 74:3275 doi:10.1002/(SICI)1097-4628(19991220)74:13<3275::AID-APP31>3.0.CO;2-2
Crank J (1975) Mathematics of diffusion, 2nd ed. Oxford University Press, Oxford
Angot A (1965) Complements de mathematiques, Cinquieme Edition, Collection technique scientifique du CNET
Gasser MS, Morad GA, Aly HF (2006) Adsorption 12:65 doi:10.1007/s10450-006-0139-y
Gercel O, Ozcan AS, Gercel HF (2007) Appl Surf Sci 253:4843 doi:10.1016/j.apsusc.2006.10.053
Boyd GE, Soldano BA (1953) J Am Chem Soc 75:6105 doi:10.1021/ja01120a003
Ruvolo-Filho A, Curti PS (2006) Ind Eng Chem Res 45:7985 doi:10.1021/ie060528y
Nikolaev AV, Logvinenko VA, Gorbatchov VM, Myachina LA (1974) Thermal Analysis. In: Buzas I (ed) Proc. Fourth ICTA, Budapest Vol. 1. p 47
Agrawal RK (1986) J Therm Anal 31:73 doi:10.1007/BF01913888
Vlaev LT, Georgieva VG, Genieva SD (2007) Oxid Comm 30:19
Zmijewski T, Pysiak J (1974) Thermal analysis. In: Buzas I (ed) Processing of the Fourth ICTA, Budapest Vol. 1. p 205
Dombrowsky NM (1968) Kinetika I Kataliz 9:251 in Russian
Agrawal KR (1986) J Therm Anal 31:73 doi:10.1007/BF01913888
Malik UR, Hasany SM, Subhani MS (2005) Talanta 66:166 doi:10.1016/j.talanta.2004.11.013
Akhtar M, Hasany SM, Bhanger MI, Iqbal S (2007) Chemosphere 66:1829 doi:10.1016/j.chemosphere.2006.09.006
Li X, Tabil LG, Panigrahi S (2007) J Polym Environ 15:25 doi:10.1007/s10924-006-0042-3
Little LH (1969) Infrared spectra of adsorbed species. Mir, Moscow
Kiselev AV, Lygin VI (1972) Infrared spectra surface compounds. Nauka, Moscow
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlaev, L., Turmanova, S. & Dimitrova, A. Kinetics and thermodynamics of water adsorption onto rice husks ash filled polypropene composites during soaking. J Polym Res 16, 151–164 (2009). https://doi.org/10.1007/s10965-008-9213-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9213-3