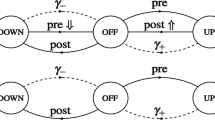
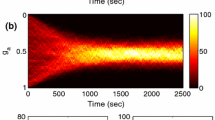
Abstract
This paper addresses two questions in the context of neuronal networks dynamics, using methods from dynamical systems theory and statistical physics: (i) How to characterize the statistical properties of sequences of action potentials (“spike trains”) produced by neuronal networks? and; (ii) what are the effects of synaptic plasticity on these statistics? We introduce a framework in which spike trains are associated to a coding of membrane potential trajectories, and actually, constitute a symbolic coding in important explicit examples (the so-called gIF models). On this basis, we use the thermodynamic formalism from ergodic theory to show how Gibbs distributions are natural probability measures to describe the statistics of spike trains, given the empirical averages of prescribed quantities. As a second result, we show that Gibbs distributions naturally arise when considering “slow” synaptic plasticity rules where the characteristic time for synapse adaptation is quite longer than the characteristic time for neurons dynamics.
Similar content being viewed by others
References
Adrian, E., Zotterman, Y.: The impulses produced by sensory nerve endings: Part II: The response of a single end organ. J. Physiol. (Lond.) 61, 151–71 (1926)
Amit, D.J.: Modeling Brain Function—the World of Attractor Neural Networks. Cambridge University Press, Cambridge (1989)
Arabzadeh, E., Panzeri, S., Diamond, M.: Deciphering the spike train of a sensory neuron: Counts and temporal patterns in the rat whisker pathway. J. Neurosci. 26(36), 9216–9226 (2006)
Artola, A., Bröcher, S., Singer, W.: Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 347(6288), 69–72 (1990)
Barbieri, R., Frank, L.M., Nguyen, D.P., Quirk, M.C., Wilson, M.A., Brown, E.N.: Dynamic analyses of information encoding in neural ensembles. Neural Comput. 16, 277–307 (2004)
Beck, C., Schloegl, F.: Thermodynamics of Chaotic Systems: An Introduction. Cambridge University Press, Cambridge (1995)
Bi, G., Poo, M.: Synaptic modification by correlated activity: Hebb’s postulate revisited. Ann. Rev. Neurosci. 24, 139–166 (2001)
Bienenstock, E.L., Cooper, L., Munroe, P.: Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2(1), 32–48 (1982)
Blanchard, P., Cessac, B., Krueger, T.: What can one learn about self-organized criticality from dynamical system theory? J. Stat. Phys. 98, 375–404 (2000)
Bliss, T., Gardner-Medwin, A.: Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetised rabbit following stimulation of the perforant path. J. Physiol. 232, 357–374 (1973)
Bohte, S.M., Mozer, M.C.: Reducing the variability of neural responses: a computational theory of spike-timing-dependent plasticity. Neural Comput. 19(2), 371–403 (2007)
Bowen, R.: Equilibrium States and the Ergodic Theory of Anosov Diffeomorphisms, vol. 470. Springer, New York (1975)
Bowen, R.: Equilibrium States and the Ergodic Theory of Anosov Diffeomorphisms, Revised Version. Springer, New York (2008)
Brette, R., Gerstner, W.: Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. J. Neurophysiol. 94, 3637–3642 (2005)
Cessac, B.: Does the complex susceptibility of the Hénon map have a pole in the upper-half plane? A numerical investigation. Nonlinearity 20, 2883–2895 (2007)
Cessac, B.: A discrete time neural network model with spiking neurons. Rigorous results on the spontaneous dynamics. J. Math. Biol. 56(3), 311–345 (2008)
Cessac, B., Samuelides, M.: From neuron to neural networks dynamics. EPJ Spec. Top. Top. Dyn. Neural Netw. 142(1), 7–88 (2007)
Cessac, B., Viéville, T.: On dynamics of integrate-and-fire neural networks with adaptive conductances. Front. Neurosci. 2(2) (2008)
Cessac, B., Blanchard, P., Krueger, T., Meunier, J.: Self-organized criticality and thermodynamic formalism. J. Stat. Phys. 115(516), 1283–1326 (2004)
Cessac, B., Vasquez, J., Viéville, T.: Parametric estimation of spike train statistics (2009, submitted)
Chazottes, J.: Entropie relative, dynamique symbolique et turbulence. Unpublished doctoral dissertation, Université de Provence—Aix Marseille I (1999)
Chazottes, J., Floriani, E., Lima, R.: Relative entropy and identification of Gibbs measures in dynamical systems. J. Stat. Phys. 90(3–4), 697–725 (1998)
Chazottes, J., Keller, G.: Pressure and equilibrium states in ergodic theory. In: Ergodic Theory. Springer, New York (2009)
Chechik, G.: Spike-timing-dependent plasticity and relevant mutual information maximization. Neural Comput. 15(7), 1481–1510 (2003)
Collet, P., Galves, A., Lopez, A.: Maximum likelihood and minimum entropy identification of grammars. Random Comput. Dyn. 3(3/4), 241–250 (1995)
Comets, F.: Detecting phase transition for Gibbs measures. Ann. Appl. Probab. 7(2), 545–563 (1997)
Cooper, L., Intrator, N., Blais, B., Shouval, H.: Theory of Cortical Plasticity. World Scientific, Singapore (2004)
Cronin, J.: Mathematical Aspects of Hodgkin-Huxley Theory. Cambridge University Press, Cambridge (1987)
Daucé, E., Quoy, M., Cessac, B., Doyon, B., Samuelides, M.: Self-organization and dynamics reduction in recurrent networks: stimulus presentation and learning. Neural Netw. 11, 521–33 (1998)
Dayan, P., Abbott, L.F.: Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. MIT Press, Cambridge (2001)
Dayan, P., Hausser, M.: In: Thrun, S., Saul, L., Schoelkopf, B. (eds.) Plasticity Kernels and Temporal Statistics, vol. 16. MIT Press, Cambridge (2004)
Delorme, A., Perrinet, L., Thorpe, S.: Networks of integrate-and-fire neurons using rank order coding b: spike timing dependent plasticity and emergence of orientation selectivity. Neurocomputing 38(40), 539–45 (2001)
Dudek, S., Bear, M.F.: Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J. Neurosci. 13(7), 2910–2918 (1993)
FitzHugh, R.: Mathematical models of threshold phenomena in the nerve membrane. Bull. Math. Biophys. 17, 257–278 (1955)
FitzHugh, R.: Impulses and physiological states in models of nerve membrane. Biophys. J. 1, 445–466 (1961)
Gao, Y., Kontoyiannis, I., Bienenstock, E.: Estimating the entropy of binary time series: methodology, some theory and a simulation study. Entropy 10(2), 71–99 (2008)
Georgeopoulos, A.P., Merchant, H., Naselaris, T., Amirikian, B.: Mapping of the preferred direction in the motor cortex. PNAS 104(26), 11068–11072 (2007)
Georgopoulos, A., Kalaska, J., Caminiti, R., Massey, J.: On the relations between the direction of two-dimensional arm movements and cell discharge in primary motor cortex. J. Neurosci. 2, 1527–1537 (1982)
Gerstner, W., Kistler, W.: Spiking Neuron Models. Cambridge University Press, Cambridge (2002)
Gerstner, W., Kistler, W.M.: Mathematical formulations of Hebbian learning. Biol. Cybern. 87, 404–415 (2002)
Grammont, F., Riehle, A.: Precise spike synchronization in monkey motor cortex involved in preparation for movement. Exp. Brain Res. 128, 118–122 (1999)
Grammont, F., Riehle, A.: Spike synchronization and firing rate in a population of motor cortical neurons in relation to movement direction and reaction time. Biol. Cybern. 88, 360–373 (2003)
Guckenheimer, J., Labouriau, I.S.: Bifurcation of the Hodgkin-Huxley equations: a new twist. Bull. Math. Biol. 55, 937–952 (1993)
Hebb, D.: The Organization of Behavior: A Neuropsychological Theory. Wiley, New York (1949)
Hirsch, M.: Convergent activation dynamics in continuous time networks. Neural Netw. 2, 331–349 (1989)
Hodgkin, A., Huxley, A.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952)
Izhikevich, E.: Simple model of spiking neurons. IEEE Trans. Neural Netw. 14(6), 1569–1572 (2003)
Izhikevich, E.: Which model to use for cortical spiking neurons? IEEE Trans. Neural Netw. 15(5), 1063–1070 (2004)
Izhikevich, E., Desai, N.: Relating stdp to bcm. Neural Comput. 15, 1511–1523 (2003)
Jaynes, E.: Information theory and statistical mechanics. Phys. Rev. 106, 620 (1957)
Ji, C.: Estimating functionals of one-dimensional Gibbs states. Probab. Theory Relat. Fields 82(2), 155–175 (1989)
Johnson, D.: Sensory discrimination: neural processes preceding discrimination decision. J. Neurophysiol. 4(6), 1793–1815 (1980)
Johnson, D.: Neural population structure and consequences for neural coding. J. Comput. Neurosci. 16(1), 69–80 (2004)
Jolivet, R., Rauch, A., Lescher, H.R., Gerstner, W.: Integrate-and-Fire Models with Adaptation Are Good Enough. MIT Press, Cambridge (2006)
Kang, K., Amari, S.I.: Discrimination with spike times and isi distributions. Neural Comput. 20, 1411–1426 (2008)
Katok, A., Hasselblatt, B.: Introduction to the Modern Theory of Dynamical Systems. Kluwer Academic, Dordrecht (1998)
Keller, G.: Equilibrium States in Ergodic Theory. Cambridge University Press, Cambridge (1998)
Levy, W., Stewart, D.: Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience 8(4), 791–797 (1983)
Malenka, R.C., Nicoll, R.A.: Long-term potentiation—a decade of progress? Science 285(5435), 1870–1874 (1999)
von-der Malsburg, C.: Self-organisation of orientation sensitive cells in the striate cortex. Kybernetik 14, 85–100 (1973)
Markram, H., Lübke, J., Frotscher, M., Sakmann, B.: Regulation of synaptic efficacy by coincidence of postsynaptic ap and epsp. Science 275, 213 (1997)
Meyer, D.: The Ruelle-Araki Transfer Operator in Classical Statistical Mechanics. Lecture Notes in Physics, vol. 123. Springer, Berlin (1980)
Miller, K., Keller, J., Stryker, M.: Ocular dominance column development: analysis and simulation. Science 245(4918), 605–615 (1989)
Nagumo, J., Arimoto, S., Yoshizawa, S.: An active pulse transmission line simulating nerve axon. Proc. IRE 50, 2061–2070 (1962)
Nemenman, I., Lewen, G., Bialek, W., de Ruyter van Steveninck, R.: Neural coding of a natural stimulus ensemble: Information at sub-millisecond resolution. PLoS Comput. Biol. 4, e1000025 (2006)
Nirenberg, S., Latham, P.: Decoding neuronal spike trains: how important are correlations. Proc. Nat. Acad. Sci. 100(12), 7348–7353 (2003)
Osbone, L., Palmer, S., Lisberger, S., Bialek, W.: Combinatorial coding in neural populations. arXiv:0803.3837 (2008)
Parry, W., Pollicott, M.: Zeta Functions and the Periodic Orbit Structure of Hyperbolic Dynamics, vols. 187–188. Asterisque (1990)
Perrinet, L., Delorme, A., Samuelides, M., Thorpe, S.: Networks of integrate-and-fire neuron using rank order coding a: how to implement spike time dependent Hebbian plasticity. Neurocomputing 38 (2001)
Rao, R., Sejnowski, T.J.: In: Solla, S., Leen, T., Muller, K. (eds.) Predictive Sequence Learning in Recurrent Neocortical Circuits, vol. 12. MIT Press, Cambridge (1991)
Rao, R., Sejnowski, T.J.: Spike-timing-dependent Hebbian plasticity as temporal difference learning. Neural Comput. 13(10), 2221–2237 (2001)
Rieke, F., Warland, D., de Ruyter van Steveninck, R., Bialek, W.: Spikes, Exploring the Neural Code. MIT Press, Cambridge (1996)
Rostro-Gonzalez, H., Cessac, B., Vasquez, J., Viéville, T.: Back-engineering of spiking neural networks parameters. J. Comput. Neurosci. (2009, submitted)
Rudolph, M., Destexhe, A.: Analytical integrate and fire neuron models with conductance-based dynamics for event driven simulation strategies. Neural Comput. 18, 2146–2210 (2006)
Ruelle, D.: Statistical Mechanics: Rigorous Results. Benjamin, New York (1969)
Ruelle, D.: Smooth dynamics and new theoretical ideas in nonequilibrium statistical mechanics. J. Stat. Phys. 95, 393–468 (1999)
Samuelides, M., Cessac, B.: Random recurrent neural networks. Eur. Phys. J., Spec. Top. 142, 7–88 (2007)
Schneidman, E., Berry, M., Segev, R., Bialek, W.: Weak pairwise correlations imply string correlated network states in a neural population. Nature 440, 1007–1012 (2006)
Sinanović, A., Johnson, D.: Toward a theory of information processing. Signal Process. 87(6), 1326–1344 (2007)
Siri, B., Berry, H., Cessac, B., Delord, B., Quoy, M.: Effects of Hebbian learning on the dynamics and structure of random networks with inhibitory and excitatory neurons. J. Physiol. Paris 101(1–3), 138–150 (2007). arXiv:0706.2602
Siri, B., Berry, H., Cessac, B., Delord, B., Quoy, M.: A mathematical analysis of the effects of Hebbian learning rules on the dynamics and structure of discrete-time random recurrent neural networks. Neural Comput. 20(12), 12 (2008). arXiv:0705.3690v1
Soula, H.: Dynamique et plasticité dans les réseaux de neurones à impulsions. Unpublished doctoral dissertation, INSA Lyon (2005)
Soula, H., Beslon, G., Mazet, O.: Spontaneous dynamics of asymmetric random recurrent spiking neural networks. Neural Comput. 18, 1 (2006)
Soula, H., Chow, C.C.: Stochastic dynamics of a finite-size spiking neural networks. Neural Comput. 19, 3262–3292 (2007)
Theunissen, F., Miller, J.: Temporal encoding in nervous systems: a rigorous definition. J. Comput. Neurosci. 2, 149–162 (1995)
Tkacik, G., Schneidman, E., Berry, M., Bialek, W.: Ising models for networks of real neurons. arXiv:q-bio/0611072 (2006)
Touboul, J.: Bifurcation analysis of a general class of nonlinear integrate-and-fire neurons. SIAM J. Appl. Math. 68(4), 1045–1079 (2008)
Toyoizumi, T., Pfister, J.-P., Aihara, K., Gerstner, W.: Generalized Bienenstock-Cooper-Munroe rule for spiking neurons that maximizes information transmission. Proc. Nat. Acad. Sci. 102, 5239–5244 (2005)
Toyoizumi, T., Pfister, J.-P., Aihara, K., Gerstner, W.: Optimality model of unsupervised spike-timing dependent plasticity: synaptic memory and weight distribution. Neural Comput. 19, 639–671 (2007)
Wood, F., Roth, S., Black, M.: Modeling neural population spiking activity with Gibbs distributions. In: Weiss, Y., Schölkopf, B., Platt, J. (eds.) Advances in Neural Information Processing Systems, vol. 18, pp. 1537–1544. MIT Press, Cambridge (2006)
Zou, Q.: Modèles computationnels de la plasticité impulsionnelle: synapses, neurones et circuits. Unpublished doctoral dissertation, Université Paris VI (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cessac, B., Rostro, H., Vasquez, J.C. et al. How Gibbs Distributions May Naturally Arise from Synaptic Adaptation Mechanisms. A Model-Based Argumentation. J Stat Phys 136, 565–602 (2009). https://doi.org/10.1007/s10955-009-9786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10955-009-9786-1