Abstract
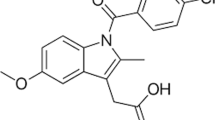
The molar solubility of bosentan (BST) in mixtures of ethylene glycol (EG) + water at different temperatures from 293.15 to 313.15 K was measured by using the shake flask technique. Experimental solubility increased with increasing temperature and mass fraction of cosolvent; so that, the largest solubility was found in neat cosolvent at 313.15 K. The cosolvency models of Jouyban–Acree and its combined version with van’t Hoff equation (Jouyban–Acree–van’t Hoff model) were used for correlation of BST solubility data with the low values of mean relative deviations (MRD% ≤ 8.1). Furthermore, some untested data were predicted based on the achieved trained models at 298.15 K. X-ray powder diffraction was also served to analyze the equilibrium solid phase crystal of BST and the results turns out that no polymorphic transformation, solvate formation or crystal transition during the whole solvent crystallization process.




Similar content being viewed by others
References
Martinez, F., Jouyban, A., Acree, W.E., Jr.: Pharmaceuticals solubility is still nowadays widely studied everywhere. Pharm. Sci. 23, 1–2 (2017)
Jouyban, A.: Handbook of Solubility data for Pharmaceuticals. CRC Press, BocaRaton FL (2010)
Barzegar-Jalali, M., Jafari, P., Jouyban, A.: Experimental determination and correlation of naproxen solubility in biodegradable low-toxic betaine-based deep eutectic solvents and water mixtures at 293.15 K to 313.15 K. Fluid Phase Equilib. (2022). https://doi.org/10.1016/j.fluid.2022.113508
Yin, H., Zhao, H., Zhao, Y.: Propylthiouracil solubility in aqueous solutions of ethylene glycol, N, N-dimethylformamide, N-methyl-2-pyrrolidone, and dimethylsulfoxide: measurement and thermodynamic modeling. J. Chem. Eng. Data. 64, 2836–2842 (2019)
Kolář, P., Shen, J.-W., Tsuboi, A., Ishikawa, T.: Solvent selection for pharmaceuticals. Fluid Phase Equilib. 194–197, 771–782 (2002)
Rubino, J.T., Boylan, J.C.: Cosolvents and cosolvency. In: Encyclopedia of Pharmaceutical Technology. Marcel Dekker, New York (1988)
Aulton, M.E.: Pharmaceutics. In: The Science of Dosage Forms Design. Churchill Livingstone, London (2002)
Vachiery, J.L.: Pulmonary arterial hypertension management—A new approach for a rare disease. Eur. Respir. Pulmon. Dis. 2, 1–50 (2016)
Krupa, A., Majda, D., Mozgawa, W., Szlęk, J., Jachowicz, R.: Physicochemical properties of bosentan and selected pde-5 inhibitors in the design of drugs for rare diseases. AAPS Pharm. Sci. Tech. 18, 1318–1331 (2017)
Azim, M.S., Husain, A., Mitra, M., Bhasin, P.S.: Pharmacological and pharmaceutical profile of bosentan: a review. Am. J. Pharm. Tech. Res. 4, 135–147 (2012)
Dangre, P.V., Sormare, V.B., Godbole, M.D.: Improvement in dissolution of bosentan monohydrate by solid dispersions using spray drying technique. Open Pharm. Sci. J. 4, 23–31 (2017)
Mohammad Shafi, MSh., Saudagar, R.B.: Solubility enhancement bosentan monohydrate using mixed hydrotropy. Int J Inst. Pharm. Life Sci. 5(3), 319–330 (2015)
Bab, R.S., Middha, A.K., Kishore, D.V., Karunakranth, D.: Development and evaluation of bosentan monohydrate loaded lipid solid dispersions. Indian J. Res. Pharm. Biotechnol. 5(6), 379–386 (2017)
Negendra, R.J.V.V., Jagan Mohan, R.N.V.V., Prasanna, M.L., Narendra, D.: Devanaboyina N.: Formulation and evaluation of bosentan nanosuspensions by nanopercipitaion methode. Int. J. Pharm. 7(1), 94–100 (2017)
Kendre, P.N., Chaudhari, P.D.: Effect of amphiphilic graft co-polymer-carrier on physical stability of bosentan nanocomposite: assessment of solubility, dissolution and bioavailability. Eur. J. Pharm. Biopharm. 126, 177–186 (2018)
Tenjarla, S.: Microemulsions: an overview and pharmaceutical applications. Crit. Rev. Ther. Drug Carrier Syst. 16, 461–521 (1999)
Serajuddin, A.T.: Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 59, 603–616 (2007)
Sajedi-Amin, S., Barzegar-Jalali, M., Fathi-Azarbayjani, A., Kebriaeezadeh, A., Martínez, F., Jouyban, A.: Solubilization of bosentan using ethanol as a pharmaceutical cosolvent. J. Mol. Liq. 232, 152–158 (2017)
Barzegar-Jalali, M., Mirheydari, S.N., Rahimpour, E., Shekaari, H., Martinez, F., Jouyban, A.: Experimental determination and correlation of bosentan solubility in (PEG 200 + water) mixtures at T = (293.15–313.15) K. Phys. Chem. Liq. 57, 504–515 (2019)
Babaei, M., Shayanfar, A., Rahimpour, E., Barzegar-Jalali, M., Martinez, F., Jouyban, A.: Solubility of bosentan in propylene glycol + water mixtures at various temperatures: experimental data and mathematical modelling. Phys. Chem. Liq. 7, 338–348 (2019)
Shakeel, F., AlAjmi, M.F., Haq, N., Siddiqui, N.A., Alam, P., Al-Rehaily, A.J.: Solubility and thermodynamic function of a bioactive compound bergenin in various pharmaceutically acceptable neat solvents at different temperatures. J. Chem. Thermodyn. 101, 19–24 (2016)
Jouyban, A., Acree, W.E., Jr., Fakhree, M.A.: Toxicity and Drug Testing. InTech Publisher, New York (2012)
Grant, D.J.W., Mehdizadeh, M., Chow, A.H.L., Fairbrother, J.E.: Non-linear van’t Hoff solubility-temperature plots and their pharmaceutical interpretation. Int. J. Pharm. 18, 25–38 (1984)
Jouyban, A., Acree, W.E., Jr.: Mathematical derivation of the Jouyban–Acree model to represent solute solubility data in mixed solvents at various temperatures. J. Mol. Liq. 256, 541–547 (2018)
Jouyban, A., Khoubnasabjafari, M., Chan, H.K., Acree, W.E., Jr.: Mathematical representation of solubility of amino acids in binary aqueous-organic solvent mixtures at various temperatures using the Jouyban-Acree model. Pharmazie 61, 789–792 (2006)
Jouyban, A., Shokri, J., Barzegar-Jalali, M., Hassanzadeh, D., Acree, W.E., Jr., Ghafourian, T., Nokhodchi, A.: Solubility of chlordiazepoxide, diazepam, and lorazepam in ethanol + water mixtures at 303.2 K. J. Chem. Eng. Data 54, 2142–2145 (2009)
Jouyban, A., Acree, W.E., Jr.: Comments on “solubility of ethyl maltol in aqueous ethanol mixtures” (Liu, B.-S.; Liu, R.-J.; Hu, Y.-Q.; Hu, Q.-F. J. Chem. Eng. Data 2008, 53, 2712−2714). J. Chem. Eng. Data 54, 1168–1170 (2009)
Ahmadian, S., Panahi-Azar, V., Fakhree, M.A.A., Acree, W.E., Jr., Jouyban, A.: Solubility of phenothiazine in water, ethanol, and propylene glycol at (298.2–338.2) K and their binary and ternary mixtures at 298.2 K. J. Chem. Eng. Data 56, 4352–4355 (2011)
Fakhree, M.A.A., Ahmadian, S., Panahi-Azar, V., Acree, W.E., Jr., Jouyban, A.: Solubility of 2-hydroxybenzoic acid in water, 1-propanol, 2-propanol, and 2-propanone at (298.2–338.2) K and their aqueous binary mixtures at 298.2 K. J. Chem. Eng. Data 57, 3303–3307 (2012)
Fathi-Azarbayjani, A., Abbasi, M., Vaez-Gharamaleki, J., Jouyban, A.: Measurement and correlation of deferiprone solubility: investigation of solubility parameter and application of van’t Hoff equation and Jouyban-Acree model. J. Mol. Liq. 215, 339–344 (2016)
Jouyban, A.: Review of the cosolvency models for predicting drug solubility in solvent mixtures: an update. J. Pharm. Pharm. Sci. 22, 466–485 (2019)
Dadmand, S., Kamari, F., Acree, W.E., Jr., Jouyban, A.: solubility prediction of drugs in binary solvent mixtures at various temperatures using a minimum number of experimental data points. AAPS Pharm. Sci. Tech. 20, 10 (2018)
Acknowledgements
Research reported in this publication was supported by Elite Researcher Grant Committee under grant number 943632 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moradi, M., Rahimpour, E., Jafari, P. et al. Solubility Measurement and Mathematical Modeling for Bosentan in Mixtures of Ethylene Glycol and Water at 293.15–313.15 K. J Solution Chem 52, 218–227 (2023). https://doi.org/10.1007/s10953-022-01227-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-022-01227-2