Abstract
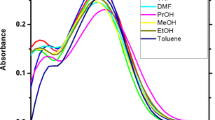
The various methods for studying polarities are based on the use of probe molecules, whose molecular spectral profile is significantly affected by the polarity of the medium. The absorption and emission spectra and dipole moments (µ g and µ e) of 2-(3-oxo-3H-benzo[f]chromen-1-ylmethoxy)-benzoic acid methyl ester (2BME) are studied in solvents of different polarities at room temperature. The determination of dipole moments by solvatochromic shift using various relations and the change in dipole moment (Δµ) were determined using Stokes shift with the variation of the solvent polarity parameter (E NT ). The value of µ e greater than µ g indicating that the probe is more polar in the higher state. DFT and TDDFT theoretical analysis of dipole moment in the vacuum and with solvent, solvent accessible surface (SAS) and molecular electrostatic potential (MEP) are also performed.













Similar content being viewed by others
References
Puttaraju, K.B., Shivashankar, K., Mahendra, M.C., Rasal, V.P., Vivek, P.N.V., Rai, K., Chanu, M.B.: Microwave assisted synthesis of dihydrobenzo[4,5] imidazo[1,2-a]pyrimidin-4-ones; synthesis, in vitro antimicrobial and anticancer activities of novel coumarin substituted dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones. Eur. J. Med. Chem. 69, 316–322 (2013)
Bouckaert, C., Serra, S., Rondelet, G., Dolusic, E., Wouters, J., Dogne, J.M., Frederick, R., Pochet, L.: Synthesis, evaluation and structure–activity relationship of new 3-carboxamide coumarins as FXIIa inhibitors. Eur. J. Med. Chem. 110, 181–194 (2016)
Farley, C.M., Dibwe, D.F., Ueda, J.Y., Hall, E.A., Awale, S., Magolan, J.: Evaluation of synthetic coumarins for antiausterity cytotoxicity against pancreatic cancers. Med. Chem. Lett 26, 1471–1474 (2016)
Ali, M.Y., Jannat, S., Jung, H.A., Choi, R.J., Roy, A., Choi, J.S.: Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure–activity analysis. Asian Pac. J. Trop. Med 9, 103–111 (2016)
Wei, W., Wu, X.W., Deng, G.G., Yang, X.W.: Anti-inflammatory coumarins with short- and long-chain hydrophobic groups from roots of Angelica dahurica cv. Hangbaizhi. Phytochem 123, 58–68 (2016)
Thakur, A., Singla, R., Jaitak, V.: Coumarins as anticancer agents: a review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 101, 476–495 (2015)
Abdelhamid, H.N., Wu, H.F.: A method to detect metal–drug complexes and their interactions with pathogenic bacteria via graphene nanosheet assist laser desorption/ionization mass spectrometry and biosensors. Anal. Chim. Acta 751, 94–104 (2012)
Abdelhamid, H.N., Khan, M.S., Wu, H.-F.: Design, characterization and applications of new ionic liquid matrices for multifunctional analysis of biomolecules: A novel strategy for pathogenic bacteria biosensing. Anal. Chim. Acta 823, 51–60 (2014)
Matagai, N., Kaifu, Y., Koizumi, M.: Solvent effects upon fluorescence spectra and the dipole moment of the excited molecules. Bull. Chem. Soc. Jpn. 29, 465–470 (1956)
Chemla, D.S., Zyss, J.: Non-linear optical properties of organic molecules and crystals. Academic Press, New York (1987)
Haley, L.V., Hameka, H.F.: Calculation of molecular electric polarizabilities and dipole moments. II. The LiH molecule. Int. J. Quantum Chem. 11, 733–741 (1977)
Nadaf, Y.F., Deshapande, D.K., Karguppikar, A.M., Inamdar, S.R.: Estimation of excited state dipole moments of exalite dyes by solvatochromic shift studies. J. Photosci. 9, 29–32 (2002)
Nadaf, Y.F., Mulimani, B.G., Inamdar, S.R.: Ground and excited state dipole moments of some exalite UV laser dyes from solvatochromic method using solvent polarity parameters. J. Mol. Struct. Theochem. 678, 177–181 (2004)
Raikar, U.S., Renuka, C.G., Nadaf, Y.F., Mulimani, B.G.: Steady-state, time-resolved fluorescence polarization behaviour and determination of dipole moments of coumarin laser dye. J. Mol. Struct. 787, 127–130 (2006)
Raikar, U.S., Renuka, C.G., Nadaf, Y.F., Mulimani, B.G., Karguppikar, A.M., Soudagar, M.K.: Solvent effects on the absorption and fluorescence spectra of coumarins 6 and 7 molecules: Determination of ground and excited state dipole moment. Spectrochim. Acta A 65, 673–677 (2006)
Katritzky, A.R., Fara, D.C., Yang, H., Tamm, K., Tamm, T., Karelson, M.: Quantitative measures of solvent polarity. Chem. Rev. 104, 175–198 (2004)
Thipperudrappa, J.: Study of solvent effect in 2,5-dpapmc dye using different solvent polarity parameters and estimation of dipole moments. Mapana. J. Sci. 14, 103–119 (2014)
Guadarrama, P., Teran, G., Ramos, E., Gutierrez, J., Hernandez, M.: Novel push–pull dendrons with high excited state dipole moments. Synthesis and theoretical analysis of unusual branched electron distribution. J. Mol. Struct. 1086, 17–24 (2015)
Joshi, S., Kumara, S., Bhattacharjee, R., Sarmah, A., Sakhuja, R., Pant, D.D.: Experimental and theoretical study: determination of dipole moment of synthesized coumarin–triazole derivatives and application as turn off fluorescence sensor: High sensitivity for iron(III) ions. Sensors Actuators B: Chem. 220, 1266–1278 (2015)
El-Daly, S.A.: Alamry. K.A.: Spectroscopic investigation and photophysics of a D-π-A-π-D type styryl pyrazine derivative. J. Fluoresc. 26, 163–176 (2016)
Joshi, S., Pant, D.D.: Solvatochromic shift and estimation of dipole moment of quinine sulfate. J. Mol. Liq. 166, 49–52 (2012)
Sidir, I., Sidir, Y.G.: Solvent effect on the absorption and fluorescence spectra of 7-acetoxy-6-(2,3-dibromopropyl)-4,8-dimethylcoumarin: determination of ground and excited state dipole moments. Spectrochim. Acta A 102, 286–296 (2013)
Zakerhamidi, M.S., Ahmadi Kandjani, S., Moghadam, M., Ortyl, E., Kucharski, S.: Substituent and solvent effects on the dipole moments and photophysical properties of two azo sulfonamide dyes. J. Mol. Struct. 996, 95–100 (2011)
Gilani, G., Hosseini, S.E., Moghadam, M., Alizadeh, E.: Excited state electric dipole moment of Nile blue and brilliant cresyl blue: A comparative study. Spectrochim. Acta A 89, 231–237 (2012)
Lackowicz, J.R.: Principles of Fluorescence Spectroscopy. Plenum Press, New York (1983)
Mataga, N., Kaifu, Y., Koizumi, M.: Solvent effects upon fluorescence spectra and the dipole moment of the excited molecules. Bull. Chem. Soc. Jpn. 29, 465–470 (1956)
Kamlet, M.J.: An examination of linear solvation energy relationships. Prog. Phys. Org. Chem. 13, 485–492 (1982)
Kamlet, M.J., Abboud, J.L.M., Taft, R.W.: The solvatochromic comparison method. 6. The π* scale of solvent polarities. J. Am. Chem. Soc. 99, 6027–6035 (1977)
Kamlet, M.J., Abboud, J.L.M., Abraham, M.H., Taft, R.W.: Linear solvation energy relationships. A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 48, 2877–2887 (1983)
Bakshiev, N.G.: Universal intermolecular interactions and their effect on the position of the electronic spectra of molecules in two component solutions Opt. Specrtrosc 16, 821–832 (1964)
Kawski, A.: Effect of polar molecules on electronic spectrum on 4-amino-phthalimide. Acta Phys. Polon. 25, 285–290 (1964)
Kawski, A.: Abnormal Stokes shift of the absorption and of the fluorescence maximum of 4-aminophthalimide in dioxane–water mixtures. Acta Phys. Polon. 28, 647–652 (1965)
Kawski, A., Stefanowska, U.: The anomalous red shift of the absorption and fluorescence spectra of 4-aminophthalimide in dependence on the ratio of homo- and heteropolar solvents. Acta Phys. Polon. 28, 809–822 (1965)
Kawski, A., Kołakowski, W.: Uber die temperaturbhangigkeit der absorptions—und fluoreszenzspektren von 4-amino-phthalimid. Acta Phys. Polon. 29, 177–186 (1966)
Kawski, A., Pasztor, B.: Elektrische dipolmomente von N-phenyl-α-naphthylaminimgrund-und anregungszustand. Acta Phys. Polon. 29, 187–193 (1966)
Chamma, A., Viallet, P.: Determination du moment dipolaire d’une molecule dans un etat excite singulet. Sci. Paris Ser. C 270, 1901–1904 (1970)
Reichardt, C., Welton, T.: Solvents and solvent effects in organic chemistry. Wiley-VCH Verlag GmbH and Co, weinheim (2010)
Sidir, I., Sidir, Y.G.: Estimation of ground and excited state dipole moments of oil red O by solvatochromic shift methods. Spectrochim. Acta A 135, 560–567 (2015)
Boregowda, P., Kalegowda, S., Rasal, V.P., Reddy, J., Koyye, J.E.: Synthesis and biological evaluation of 4-(3-hydroxy-benzofuran-2-yl)coumarins. Org. Chem. Int 2014, 1–7 (2014)
Reichardt, C.: Solvents and Solvent Effects in Organic Chemistry. VCH, New York (2005)
Lide, D.R. (ed.): Handbook Chemistry and Physics, 76th edn. CRC Press, Boca Raton (1995)
Lide, D.R. (ed.): Handbook Chemistry and Physics, 80th edn. CRC Press, New York (1999)
Reichardt, C., Ratajczak, H., Orville-Thomas, W.J. (eds.): Molecular Interactions, vol. 3. John Wiley and Sons Ltd, New York (1982)
Lippert, E.: Spektroskopische best immung des dipole moment saromatischer verbindungen im ersten angeregten singulettzust and. Z Eleckchem 6, 962–975 (1957)
Bakshiev, N.G.: Universal intermolecular interactions and their effect on the position of the electronic spectra of molecules in two component solutions. Opt. Spektrosk. 16, 821–832 (1964)
Chamma, A., Viallet, P.: Determination du moment dipolaired’une molecule dans un etat excite singulet. Comptes Rendus Acad. Sci. Paris Ser. C 270, 1901–1908 (1970)
McRae, E.G.: Theory of solvent effects on molecular electronic spectra frequency shifts. J. Phys. Chem. 61, 562–572 (1957)
Edward, J.T.: Molecular volumes and parachor. Chem. Ind. 30, 774–777 (1956)
Reichardt, C.: Solvents and Solvent Effects in Organic Chemistry. VCH, Weinhwim (1988)
Kamlet, M.J., Abboud, J.L.M., Taft, R.W.: An examination of linear solvation energy relationships. Prog. Phys. Org. Chem. 13, 485–630 (1981)
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, M.S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguyen, K.A., Su, S., Windus, T.L., Dupuis, M., Montgomery, J.A.: General atomic and molecular electronic structure system. J. Comput. Chem. 14, 1347–1363 (1993)
Pramanik, S., Banerjee, P., Sarkar, A., Mukherjee, A., Mahalanabis, K.K., Bhattacharya, S.C.: Spectroscopic investigation of 3-pyrazolyl 2-pyrazoline derivative in homogeneous solvents. Spectrochim. Acta A 71, 1327–1332 (2008)
Willard, D.M., Riter, R.E., Levinger, N.E.: Dynamics of polar solvation in lecithin/water/cyclohexane reverse micelles. J. Am. Chem. Soc. 120, 4151–4160 (1998)
Saha, S., Samanta, A.: Influence of the structure of the amino group and polarity of the medium on the photophysical behavior of 4-amino-1,8-naphthalimide derivatives. J. Phys. Chem. A 106, 4763–4771 (2002)
Lippert, E.: Spektroskopische bistimmung des dipolmomentes aromatischer verbindugen im ersten angeregten singulettzustand. Z Electrochem 61, 962–975 (1957)
Kamlet, M.J., Taft, R.W.: The solvatochromic comparison method. The α-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 98, 377–383 (1976)
Nagarajan, V., Brearley, A.M., Kang, T., Barbara, P.F.: Time-resolved spectroscopic measurements on microscopic solvation dynamics. J. Chem. Phys. 86, 3183–3196 (1987)
Kahlow, M.A., Kang, T.J., Berbara, P.F.: Transient solvation of polar dye molecules in polar aprotic solvent. J. Chem. Phys. 86, 2372–2378 (1988)
Khattab, M., Van Dongen, M., Feng Wang, F., Clayton, A.H.A.: Solvatochromism and linear solvation energy relationship of the kinase inhibitor SKF86002. Spectrochim. Acta A 170, 226–233 (2017)
Wong, M.W., Frisch, M.J., Wiberg, K.B.: Solvent effects the mediation of electrostatic effects by solvents. J. Am. Chem. Soc. 113, 4476–4782 (1991)
Cances, E., Mennucci, B., Tomasi, J.: A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys 107, 3032–3041 (1997)
Sıdır, I., Gulseven Sıdır, Y., Kumalar, M., Tasal, E.: Ab initio Hartree-Fock and density functional theory investigations on the conformational stability, molecular structure and vibrational spectra of 7-acetoxy-6-(2,3-dibromopropyl)-4,8-dimethylcoumarin molecule. J. Mol. Struct. 964, 134–151 (2010)
Scrocco, E., Tomasi, J.: Electronic molecular structure, reactivity and intermolecular forces: an euristic interpretation by means of electrostatic molecular potentials. Adv. Quantum Chem. 11, 115–198 (1979)
Luque, F.J., Lopez, J.M., Orozco, M.: Perspective on electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Theor. Chem. Acc. 103, 343–345 (2000)
Acknowledgements
The author Y.F. Nadaf thankful to University Grant Commission New Delhi, India, for financial assistance. YFN thankful to Dr. G. Sriprakash. MSCW for fruitful discussion. Also thankful are Mohamed Zikriya and Pramod A.G. Research Scholars Bangalore University, Bengaluru.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renuka, C.G., Shivashankar, K., Boregowda, P. et al. An Experimental and Computational Study of 2-(3-Oxo-3H-benzo[f] chromen-1-ylmethoxy)-Benzoic Acid Methyl Ester. J Solution Chem 46, 1535–1555 (2017). https://doi.org/10.1007/s10953-017-0661-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0661-4