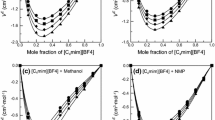
In recent years, ionic liquids have increasingly gained importance as green solvents. The potential of these organic salts, which are moisture and air stable at room temperature, for new chemical processes and technologies is beginning to be recognized. Research on the thermophysical properties of ionic liquids and their mixtures form the basis for future applications. In this contribution, densities, refractive indices, speeds of sound and dynamic viscosities of 1-methyl-3-octylimidazolium tetrafluoroborate, [Omim][BF4], the room temperature ionic liquid (IL) in binary mixtures with methanol, ethanol, 1-propanol and 2-propanol were measured at 298.15K and atmospheric pressure. The excess molar volumes and molar refraction, isentropic compressibility and dynamic viscosity changes of mixing have been calculated and were satisfactorily correlated by the Redlich–Kister polynomial.
Similar content being viewed by others
References
0. Z. Gu and and J. F. Brennecke, Volume Expansivities and Isothermal Compressibilities of Imidazolium and Pyridinium-based Ionic Liquids, J. Chem. Eng. Data 47, 339–345 (2002).
A. Heintz, D. Klasen, and J. K. Lehmann, Excess Molar Volumes and Viscosities of Binary Mixtures of Methanol and the Ionic Liquid 4-Methyl-N-butylpyridinium Tetrafluoroborate at 25, 40, and 50∘C, J. Solution Chem. 31, 467–476 (2002).
J. Wang, Y. Tian, and K. Zhuo, A Volumetric and Viscosity Study for the Mixtures of 1-n-Butyl-3methylimidazolium Tetrafluoroborate Ionic Liquid with Acetonitrile, Dichloromethane, 2-Butanone and N,N-dimethylformamide, Green Chem. 5, 618–622 (2003).
A. Heintz, J. K. Lehmann, and C. Wertz, Thermodynamic Properties of Mixtures Containing Ionic Liquids. 3. Liquid-liquid Equilibria of Binary Mixtures of 1-Ethyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide with Propan-1-ol, Butan-1-ol, and Pentan-1-ol, J. Chem. Eng. Data 48, 472–474 (2003).
J. Zhang, W. Wu, T. Jiang, H. Gao, Z. Liu, J. He, and B. Han, Conductivities and Viscosities of the Ionic Liquid [bmim][PF6] + Water + Ethanol and [bmim][PF6] + Water + Acetone Ternary Mixtures, J. Chem. Eng. Data 48, 1315–1317 (2003).
S. Zhang, X. Li, H. Chen, J. Wang, J. Zhang, and M. Zhang, Determination of Physical Properties for the Binary System of 1-Ethyl-3-methylimidazolium Tetrafluoroborate + H2O, J. Chem. Eng. Data 49, 760–764 (2004).
K. S. Kim, B. K. Shin, H. Lee, and F. Ziegler, Refractive Index and Heat Capacity of 1-Butyl-3-methylimidazolium Bromide and 1-Butyl-3-methylimidazolium Tetrafluoroborate, and Vapor Pressure of Binary Systems for 1-Butyl-3-Methylimidazolium Bromide + Trifluoroethanol and 1-Butyl-3-methylimidazolium Tetrafluoroborate + Trifluoroethanol, Fluid Phase Equil. 218, 215–220 (2004).
H. Xu, D. Zhao, P. Xu, F. Liu, and G. Gao, Conductivity and Viscosity of 1-Allyl-3-Methyl-Imidazolium Chloride + Water and + Ethanol from 293.15 K to 333.15 K, J. Chem. Eng. Data 50, 133–135 (2005).
T. V. Vasiltsova, S. P. Verevkin, E. Bich, A. Heintz, R. Bogel-Lukasik, and U. Domanska, Thermodynamic Properties of Mixtures Containing Ionic Liquids. Activity Coefficients of Ethers and Alcohols in 1-Methyl-3-Ethyl-Imidazolium Bis(trifluoromethyl-sulfonyl) Imide Using the Transpiration Method, J. Chem. Eng. Data 50, 142–148 (2005).
L. P. N. Rebelo, V. Najdanovic-Visak, Z. P. Visak, M. Nunes da Ponte, J. Szydlowski, C. A. Cerdeirina, J. Troncoso, L. Romani, J. M. S. S. Esperanca, H. J. R. Guedes, and H. C. de Sousa, A Detailed Thermodynamic Analysis of [C4mim][BF4] + Water as a Case Study to Model Ionic Liquid Aqueous Solutions, Green Chem. 6, 369–381 (2004).
J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker, and R. D. Rogers, Characterization and Comparison of Hydrophilic and Hydrophobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation, Green Chem. 3, 156–164 (2001).
K. N. Marsh, J. A. Boxall, and R. Lichtenthaler, Room Temperature Ionic Liquids and Their Mixtures—A Review, Fluid Phase Equil. 219, 93–98 (2004).
C. P. Mehnert, N. C. Dispenziere, and R. A. Cook, US Patent Application A1 20040074842 (2004).
J. Dupont, C. S. Consorti, P. A. Z. Suarez, R. F. de Souza, S. L. Fulmer, D. P. Richardson, T. E. Smith, and S. Wolff, Preparation of 1-n-Butyl-3-methylimidazolium Based Room Temperature Ionic Liquids, Org. Synth. 79, 236–243 (2002).
P. Bonhôte, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram, and M. Grätzel, Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts, Inorg. Chem. 35, 1168–1178 (1996).
J. Holbrey and K. R. Seddon, The Phase Behaviour of 1-Alkyl-3-methylimidazolium Tetrafluoroborates: Ionic Liquids and Ionic Liquid Crystals, J. Chem. Soc. Dalton Trans. 2133–2139 (1999).
L. C. Branco, J. N. Rosa, J. J. Moura Ramos, and C. A. M. Afonso, Preparation and Characterization of New Room Temperature Ionic Liquids, Chem. Eur. J. 8, 3671–3677 (2002).
K. R. Seddon, A. Stark, and M. J. Torres, Viscosity and Density of 1-alkyl-3-Methylimidazolium Ionic Liquids, ACS Symp. Ser. 819, 34–49 (2002).
T. M. Aminabhavi, M. Y. Aralaguppi, Sh. Harogoppad, and R. H. Balundgi, Densities, Viscosities, Refractive Indices, and Speeds of Sound for Methyl Acetoacetate + Aliphatic Alcohols (C1-C8), J. Chem. Eng. Data 38, 31–39 (1993).
J. A. Riddick, W. B. Bunger, and T. Sakano, Organic Solvents, 4th Edn. (John Wiley, New York, 1986).
J. George and N. V. Sastry, Densities, Dynamic Viscosities, Speeds of Sound, and Relative Permittivities for Water + Alkanediols (Propane-1,2- and -1,3-diol and Butane-1,2-, -1,3-, -1,4-, and -2,3-diol) at Different Temperatures, Chem. Eng. Data 48, 1529–1539 (2003).
K. R. Seddon, A. Stark, and M. J. Torres, Influence of Chloride, Water, and Organic Solvents on the Physical Properties of Ionic Liquids, Pure Appl. Chem. 72, 2275–2287 (2000).
D. Patterson and G. Delmas, Corresponding States Theories and Liquid Models, Disc. Faraday Soc. 49, 98–105 (1970).
I. Pregogine, The Molecular Theory of Solution (North-Holland, Amsterdam, 1957).
P. J. Flory, Statistical Thermodynamics of Liquid Mixtures, J. Am. Chem. Soc. 87, 1833–1838 (1965).
O. J. Redlich and A. T. Kister, Thermodynamics of Nonelectrolytic Solutions. Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem. 40, 345–348 (1948).
P. Bevington, Data Reduction and Error Analysis for the Physical Sciences (McGraw-Hill, New York, 1969).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arce, A., Rodil, E. & Soto, A. Physical and Excess Properties for Binary Mixtures of 1-Methyl-3-Octylimidazolium Tetrafluoroborate, [Omim][BF4], Ionic Liquid with Different Alcohols. J Solution Chem 35, 63–78 (2006). https://doi.org/10.1007/s10953-006-8939-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10953-006-8939-y