Abstract
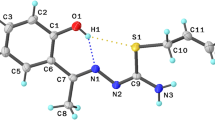
The new salts of pyridine-4-aldehyde thiosemicarbazone: perchlorate (I) and trifluoromethane sulfonate (II) HN+C5H4-CH=N-NH-C(S)-NH2·X− (X = ClO4, CF3SO3) were synthesized and studied by IR and NMR spectroscopy and X-ray diffraction analysis. The compounds were synthesized by a reaction of pyridine-4-aldehyde thiosemicarbazone with chloric or trifluoromethane sulfonic acid, respectively. Compound I crystallized in the triclinic crystal system, space group P-1, a = 6.8691(2) Å, b = 9.5406(4) Å, c = 9.6348(4) Å, α = 78.838(1)°, β = 77.618(1)°, γ = 69.661(1)°, Z = 2. Compound II crystallized in the monoclinic crystal system, space group P21/c, a = 7.3149(8) Å, b = 11.9830(16) Å, c = 15.143(2) Å, β= 96.949(4)°, Z = 4. The structures are formed by hydrogen-bonded ions. Moreover, the cations are linked in “dimmers” due to the weak N-H...S hydrogen bonds.
Similar content being viewed by others
References
T. S. Lobana, S. Khanna, R. Sharma et al., Cryst. Growth Des. (Spain), 8, No. 4, 1203–1212 (2008).
P. Souza, A. I. Matesanz, and V. Fernandez, J. Chem. Soc. Dalton Trans., 14, 3011–3014 (1996).
D. Kovala-Demertzi, N. Konrkonmelis, D. X. West, et al., Eur. J. Inorg. Chem., 6, 861–864 (1998).
V. A. Jadhav, J. Indian Chem. Soc., 72, No. 9, 651–656 (1995).
J. L. J. Dearling, J. S. Lewis, D. W. Mc Carthy, et al., Chem. Commun., 22, 2531–2535 (1998).
O. E. Offiong, Spectrochim. Acta A, 50, No. 13, 2167–2176 (1994).
A. Diaz, R. Cao, and A. Garcia, Monatsh. Chem., 125, Nos. 8/9, 823–826 (1994).
H. H. Fox, USA Patent 2676178, 1954 [Chem. Abstr., 49, 7604 (1955)].
H. H. Fox, J. Org. Chem., 17, 555–558 (1952).
V. S. Jolly and K. P. Sharma, J. Indian Chem. Soc., 67, 412–416 (1990).
W. O. Foye, A. R. Banijamali, and G. Patarapanich, J. Pharm. Sci., 75, 1180–1184 (1986).
V. E. Ivanov, N. G. Tikhomirova, A. B. Tomchin, et al., Khim.-Farm. Zh., 23, 588–592 (1989).
R. P. Gupta and N. L. Narayna, Pharm. Acta Helv., 72, 43–45 (1997).
H. Wei-xiao, Sun Nan, Yang Zhong-yu., J. Med. Chem. (China), 11, No. 3, 129–133 (2001).
M. Liu, T. Lin, P. Penketh, and A. C. Sartorelli, J. Med. Chem., 38, 4234–4240 (1995).
D. L. Klayman, J. P. Scovill, and J. F. Bartosevich, Eur. J. Med. Chem. Ther., 16, 317–320 (1981).
L. I. Larina, V. N. Elokhina, T. I. Yaroshenko, et al., Magn. Res. Chem., 45, No. 8, 667–673 (2007).
T. N. Komarova, R. V. Karnaukhova, M. V. Sigalov, et al., Izv. Akad. Nauk SSSR, Ser. Khim., 1176–1179 (1990).
A. S. Nakhmanovich, R. V. Karnaukhova, T. N. Komarova, et al., Khim. Geterotsikl. Soedin., 123–125 (1988).
R. E. Hadenbach and H. Gysin, Experientia, 8, 184–189 (1952).
A. K. Nandi, S. Chudhuri, S. K. Mazumda, et al., J. Chem. Soc. Perkin II, 1729–1733 (1964).
R. Restivo and G. J. Pakenik, Acta Crystallogr., 26, 1397–1402 (1970).
I. C. Mendes, L. R. Teixeira, R. Lima, et al., J. Mol. Struct., 559, 355–360 (2001).
L. I. Larina, R. V. Karnaukhova, A. S. Nakhmanovich, et al., ibid., 604, 165–176 (2002).
Bruker (2004). APEX2 (Version 1.08), SAINT (Version 7.03), SADABS (Version 2.11) and SHELXTL (Version 6.12). Bruker AXS Inc., Madison, WI, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2009 by A. I. Smolentsev, L. G. Lavrenova, V. N. Elokhina, A. S. Nakhmanovich, and L. I. Larina
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 50, No. 3, pp. 522–526, May–June, 2009.
Rights and permissions
About this article
Cite this article
Smolentsev, A.I., Lavrenova, L.G., Elokhina, V.N. et al. Crystal structures of pyridine-4-aldehyde thiosemicarbazone perchlorate and trifluoromethane sulfonate. J Struct Chem 50, 500–504 (2009). https://doi.org/10.1007/s10947-009-0076-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10947-009-0076-1