Abstract
Metal–organic frameworks with diverse structures and unique properties have demonstrated that can be an ideal substitute for natural enzymes in colorimetric sensing platform for analyte detection in various fields such as environmental chemistry, biotechnology and clinical diagnostics, which have attracted the scientist’s attention, recently. In this study, a porous coordination network (denoted as PCN-222) was synthesized as a new biomimetic material from an iron linked tetrakis (4-carboxyphenyl) porphyrin (named as Fe-TCPP) as a heme-like ligand and Zr6 linker as a node. This catalyst shows the peroxidase and catalase activities clearly. The mechanism of peroxidase activity for PCN-222 was investigated using the spectrophotometric methods and its activity was compared with the other nanoparticles which, the results showed a higher activity than the other catalysts. Also, the hydrogen peroxide was detected by PCN-222(Fe) based on the peroxidase-like activity. For detection of hydrogen peroxide a linear range of 3–200 µM and detection of limit (LOD) 1 µM (3σ/slope), under optimal conditions were obtained. Moreover, based on the high tendency of PCN-222(Fe) to combine with the TMB as a chromogenic substrate in the peroxidase-like activity, we developed the sensitive and selective colorimetric assay for glucose detection that was found a detection limit (LOD) of 2.2 µM in the linear range from 12 to 75 µM. Finally due to the good catalytic activity of PCN-222(Fe), it was used to detection of glucose and hydrogen peroxide in real samples.
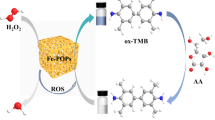
Graphical abstract











Similar content being viewed by others
References
W.P. Lustig, S. Mukherjee, N.D. Rudd, A.V. Desai, J. Li, S.K. Ghosh, Metal–organic frameworks: functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 46, 3242–3285 (2017)
J. Kim, A.S. Cambell, J. Wang, Wearable non-invasive epidermal glucose sensors: a review. Talanta 177, 163–170 (2018)
L. Guo, L. Mao, K. Huang, H. Liu, Pt–Se nanostructures with oxidase-like activity and their application in a selective colorimetric assay for mercury (II). J. Mater. Sci. 52, 10738–10750 (2017)
G. Maduraiveeran, M. Sasidharan, V. Gansan, Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 103, 113–129 (2018)
F. Bibi, C. Guillaume, N. Gontard, B. Sorli, A review: RFID technology having sensing aptitudes for food industry and their contribution to tracking and monitoring of food products. Trends Food Sci. Technol. 62, 91–103 (2017)
F. Qiao, L. Chen, X. Li, L. Li, S. Ai, Peroxidase-like activity of manganese selenide nanoparticles and its analytical application for visual detection of hydrogen peroxide and glucose. Sens. Actuator B-Chem. 193, 255–262 (2014)
W. Chen, J. Chen, Y.B. Feng, L. Hong, Q.Y. Chen, L.F. Wu, X.H. Lin, X.H. Xia (2012) Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 137, 1706–1712.
Y. Gao, Y. Wu, J. Di, Colorimetric detection of glucose based on gold nanoparticles coupled with silver nanoparticles. Spectrochim. Acta Part A. 173, 207–212 (2017)
C.L. Hsu, J.H. Lin, D.X. Hsu, S.H. Wang, S.Y. Lin, T.J. Hsueh (2017) Enhanced non-enzymatic glucose biosensor of ZnO nanowires via decorated Pt nanoparticles and illuminated with UV/green light emitting diodes. Sens. Actuators B-Chem. 238, 150–159
Y. Ding, M. Chen, K. Wu, M. Chen, L. Sun, Z. Liu, Z. Shi, Q. Liu, High-performance peroxidase mimics for rapid colorimetric detection of H2O2 and glucose derived from perylene diimides functionalized Co3O4 nanoparticles. Mater. Sci. Eng. C. 80, 558–565 (2017)
Z. Zhao, Q. Ou, X. Yin, J. Liu. Nanomaterial-based electrochemical hydrogen peroxide biosensor. Int. J. Biosens. Bioelectron. 2, 25–28 (2017)
Q. Liu, Y. Yang, X. Lv, Y. Ding, Y. Zhang, J. Jing, C. Xu, One-step synthesis of uniform nanoparticles of porphyrin functionalized ceria with promising peroxidase mimetics for H2O2 and glucose colorimetric detection. Sens. Actuator B-Chem 240, 726–734 (2017)
L.C. Clark, C. Lyons, Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N.Y. Acad. Sci. 102, 29–45 (1962)
V. Cerda, A. Gonzalez, K. Danchana, From thermometric to spectrophotometric kinetic-catalytic methods of analysis: a review. Talanta 167, 733–746 (2017)
M. Liu, R. Liu, W. Chen, Graphene wrapped Cu2O nanocubes: non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 45, 206–212 (2013)
W. Li, D. Qian, Q. Wang, Y. Li, N. Bao, H. Gu, C. Yu, Fully-drawn origami paper analytical device for electrochemical detection of glucose. Sens. Actuators B-Chem. 231, 230–238 (2016)
C. Shen, J. Su, X. Li, J. Luo, M. Yang, Electrochemical sensing platform based on Pd–Au bimetallic cluster for non-enzymatic detection of glucose. Sens. Actuators B-Chem. 209, 695–700 (2015)
F. Wang, Q. Hao, Y. Zhang, Y. Xu, W. Lei, Fluorescence quenchometric method for determination of ferric ion using boron-doped carbon dots. Microchim. Acta 183, 273–279 (2016).
Z. Yang, Z. Zhang, Y. Jiang, M. Chi, G. Nie, X. Lu, C. Wang, Palladium nanoparticles modified electrospun CoFe2O4 nanotubes with enhanced peroxidase-like activity for colorimetric detection of hydrogen peroxide. RSC Adv. 6, 33636–33642 (2016)
R.J. Russell, M.V. Pishko, C.C. Gefrides, M.J. McShane, G.L. Cote, A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly (ethylene glycol) hydrogel. Anal. Chem. 71, 3126–3132 (1999).
P.H. Hynninen, V. Kaartinen, E. Kolehmainen, Horseradish peroxidase-catalyzed oxidation of chlorophyll a with hydrogen peroxide: characterization of the products and mechanism of the reaction. Biochim. Biophys. Acta 1797, 531–542 (2010)
S. Dong, L. Mao, S. Luo, L. Zhou, Y. Feng, S. Gao. Comparison of lignin peroxidase and horseradish peroxidase for catalyzing the removal of nonylphenol from water. Environ. Sci. Pollut. Res. Int. 21, 2358–2366 (2014)
S.B. Maddinedi, B.K. Mandal, Peroxidase like activity of quinic acid stabilized copper oxide nanosheets. Austin J. Anal. Pharm. Chem. 1, 1–4 (2014)
L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett, X. Yan, Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007).
H. Wei, E. Wang, Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 42, 6060–6093 (2013)
J. Mu, Y. Wang, M. Zhao, L. Zhang, Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 48, 2540–2542 (2012)
R. Andre, F. Natalio, M. Humanes, J. Leppin, K. Heinze, R. Wever, H.C. Schroeder, W.E.G. Mueller, W. Tremel, V2O5 Nanowires with an intrinsic peroxidase-like activity. Adv. Funct. Mater. 21, 501–509 (2011)
S.K. Maji, A.K. Dutta, P. Biswas, D.N. Srivastava, P. Paul, A. Mondal, B. Adhikary, Synthesis and characterization of FeS nanoparticles obtained from a dithiocarboxylate precursor complex and their photocatalytic, electrocatalytic and biomimic. Appl. Catal. A-Gen. 419, 170–177 (2012)
Y. Song, K. Qu, C. Zhao, J. Ren, X. Qu, Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 22, 2206–2210 (2010)
W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng, Y. Huang, Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 47, 6695–6697 (2011)
J.W. Zhang, H.T. Zhang, Z.Y. Du, X. Wang, S.H. Yu, H.L. Jiang, Water-stable metal–organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem. Commun. 50, 1092–1094 (2014)
Y. Bai, Y. Dou, L.H. Xie, W. Rutledge, J.R. Li, H.C. Zhou, Zr-based metal–organic frameworks: design, synthesis, structure, and applications. Chem. Soc. Rev. 45, 2327–2367 (2016)
H. Deng, S. Grunder, K.E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A.C. Whalley, Z. Liu, S. Asahina, Large-pore apertures in a series of metal-organic frameworks. Science 336, 1018–1023 (2012)
M.D. Allendorf, V. Stavila, Crystal engineering, structure–function relationships, and the future of metal–organic frameworks. CrysEngCommun 17, 229–246 (2015)
D. Sun, W. Liu, M. Qiu, Y. Zhang, Z. Li, Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal–organic frameworks (MOFs). Chem. Commun. 51, 2056–2059 (2015)
W. Morris, B. Volosskiy, S. Demir, F. Gandara, P.L. McGrier., H. Furukawa, D. Cascio, J.F. Stoddart, O.M. Yaghi, Synthesis, structure, and metalation of two new highly porous zirconium metal–organic frameworks. Inorg. Chem. 51, 6443–6445 (2012)
Y. Chen, T. Hoang, S. Ma, Biomimetic catalysis of a porous iron-based metal–metalloporphyrin framework. Inorg. Chem. 51, 12600–12602 (2012)
X.S. Wang, M. Chrzanowski, D. Yuan, B.S. Sweeting, S. Ma, Covalent heme framework as a highly active heterogeneous biomimetic oxidation catalyst. Chem. Mater, 26, 1639–1644 (2014).
L. Ai, L. Li, C. Zhang, J. Fu, J. Jiang, MIL-53 (Fe): a metal–organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chem.–Eur. J 19, 15105–15108 (2013)
H. Yu, D. Long, Highly chemiluminescent metal-organic framework of type MIL-101 (Cr) for detection of hydrogen peroxide and pyrophosphate ions. Microchim. Acta., 183, 3151–3157 (2016).
K. Wang, D. Feng, T.F. Liu, J. Su, S. Yuan, Y.P. Chen, M. Bosch, X. Zou, H.C. Zhou, A series of highly stable mesoporous metalloporphyrin Fe-MOFs. J. Am. Chem. Soc. 136, 13983–13986 (2014)
D. Feng, W.C. Chung, Z. Wei, Z.Y. Gu, H.L. Jiang, Y.P. Chen, D.J. Darensbourg, H.C. Zhou, Construction of ultrastable porphyrin Zr metal–organic frameworks through linker elimination. J. Am. Chem. Soc. 135, 17105–17110 (2013)
D. Feng, Z.Y. Gu, J.R. Li, H.L. Jiang, Z. Wei, H.C. Zhou, Zirconium-metalloporphyrin PCN-222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. 51, 10307–10310 (2012)
P.G. Rodríguez, C.F. Batista, R. Vazquez-Duhalt, B. Valderrama. A novel heme peroxidase from Raphanus sativus intrinsically resistant to hydrogen peroxide. Eng. Life Sci. 8, 286–296 (2008)
A.J. Howarth, Y. Liu, J.T. Hupp, O.K. Farha, Metal–organic frameworks for applications in remediation of oxyanion/cation-contaminated water. Crys Eng Comm. 17, 7245–7253 (2015)
Z.Y. Gu, J. Park, A. Raiff, Z. Wei, H.C. Zhou, Metal–organic frameworks as biomimetic catalysts. Chem Cat Chem. 6, 67–75 (2014)
D.G. Blackmond, Reaction progress kinetic analysis: a powerful methodology for mechanistic studies of complex catalytic reactions. Angew. Chem., Int. Ed. 44, 4302–4320 (2005)
B.H.J. Hostee, Non-Inverted Versus Inverted Plots in Enzyme Kinetics. Nature 184, 1296–1298 (1959)
S. Kumari, B. Dhar, C. Panda, A. Meena, S. Gupta, Fe-TAML encapsulated inside mesoporous silica nanoparticles as peroxidase mimic: femtomolar protein detection. ACS Appl. Mater. Interfaces 6, 13866–13873 (2014)
E. Austin, M. Gouterman, Porphyrins XXXVII. Absorption and emission of weak complexes with acids, bases, and salts. Bioinorg. Chem. 9, 281–298 (1978)
S. Gawande, S.R. Thakare, Ternary polymer composite of graphene, carbon nitride, and poly (3-hexylthiophene): an efficient photocatalyst. Chem Cat Chem 4, 1759–1763 (2012)
E.Y. Choi, T.H. Han, J. Hong, J.E. Kim, S.H. Lee, H.W. Kim, S.O. Kim, Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 20, 1907–1912 (2010)
X.L. Lv, K. Wang, B. Wang, J. Su, X. Zou, Y. Xie, J.R. Li, H.C. Zhou, A base-resistant metalloporphyrin metal–organic framework for C–H bond halogenation. J. Am. Chem. Soc. 139, 211–217 (2017)
S. Sohrabi, S. Dehghanpour, M. Ghalekhani, A cobalt porphyrin-based metal organic framework/multi-walled carbon nanotube composite electrocatalyst for oxygen reduction and evolution reactions. J. Mater. Sci. 53, 3624–3639 (2018)
Y. Yu, P. Ju, D. Zhang, X. Han, X. Yin, L. Zheng, C. Sun, Peroxidase-like activity of FeVO4 nanobelts and its analytical application for optical detection of hydrogen peroxide. Sens. Actuator B-Chem 233, 162–172 (2016)
K. Nazari, S. Shokrollahzadeh, A. Mahmoudi, F. Mesbahi, N. Seyed Matin, A.A. Moosavi-Movahedi, Iron (III) protoporphyrin/MCM41 catalyst as a peroxidase enzyme model: preparation and typical test reactions. J. Mol. Catal. A Chem. 239, 1–9 (2005)
N.C. Veitch, Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65, 249–259 (2004)
K. Nazari, A. Mahmoudi, R. Khodafarin, A.A. Moosavi Movahedi, A. Mohebi, Stabilizing and suicide-peroxide protecting effect of Ni2+ on horseradish peroxidase. J. Iran. Chem. Soc. 2, 232–237 (2005)
A. Claiborne, I. Fridovich, Chemical and enzymic intermediates in the peroxidation of o-dianisidine by horseradish peroxidase. 1. Spectral properties of the products of dianisidine oxidation. Biochemistry 18, 2324–2329 (1979)
A. Mahmoudi, K. Nazari, A.A. Moosavi-Movahedi, A.A. Saboury, Enthalpy analysis of horseradish peroxidase in the presence of Ni2+: a stabilization study. Thermochim. Acta 385, 33–39 (2002)
Y. Liu, M. Yuan, L. Guo, R. Qiao, An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe3O4 and glucose oxidase nanocomposites. Biosens. Bioelectron. 52, 391–396 (2014)
A. Mahmoudi, K. Nazari, N. Mohammadian, A.A. Moosavi-Movahedi, Effect of Mn2+, Co2+, Ni2+, and Cu2+ on horseradish peroxidase. Appl. Biochem. Biotechnol. 104, 81–94 (2003)
J. Shu, Z.L. Qiu, Q.H. Wei, J.Y. Zhuang, D.P. Tang, Cobalt-porphyrin-platinum-functionalized reduced graphene oxide hybrid nanostructures: a novel peroxidase mimetic system for improved electrochemical immunoassay. Sci. Rep 5, 15113 (2015)
M. Khosraneh, A. Mahmoudi, H. Rahimi, K. Nazari, A.A. Moosavi-Movahedi, Suicide-Peroxide inactivation of microperoxidase-11: a kinetic study. J. Enzyme Inhibit. Med. Chem. 22, 677–684 (2007)
X. Wang, K. Qu, B. Xu, J. Ren, X. Qu, Multicolor luminescent carbon nanoparticles: synthesis, supramolecular assembly with porphyrin, intrinsic peroxidase-like catalytic activity and applications. Nano Research 4, 908–920 (2011)
Y. Sun, H.C. Zhou, Recent progress in the synthesis of metal–organic frameworks. Sci. Technol. Adv. Mater. 16, 054202 (2015)
D. Metelista, O. Rus, A. Puchkaev, Russ, Heme-containing hydroperoxide test systems for inhibitors of free-radical reactions. J. Appl. Chem. 70, 1629–1636 (1997)
M. Kim, J. Shim, T. Li, J. Lee, H. Park, Fabrication of nanoporous nanocomposites entrapping Fe3O4 magnetic nanoparticles and oxidases for colorimetric biosensing. Chem. Eur. J. 17, 10700–10707 (2011)
Q. Liu, P. Chen, Z. Xu, M. Chen, Y. Ding, K. Yue, J. Xu, A facile strategy to prepare porphyrin functionalized ZnS nanoparticles and their peroxidase-like catalytic activity for colorimetric sensor of hydrogen peroxide and glucose. Sens. Actuator B-Chem. 251, 339–348 (2017)
H.Q. Zheng, C.Y. Liu, X.Y. Zeng, J. Chen, J. Lu, R.G. Lin, R. Cao, Z.J. Lin, J.W. Su, MOF-808: a metal–organic framework with intrinsic peroxidase-like catalytic activity at neutral pH for colorimetric biosensing. Inorg. Chem. 57, 9096–9104 (2018)
H. Yang, R. Yang, P. Zhang, Y. Qin, T. Chen, F. Ye, A bimetallic (Co/2Fe) metal-organic framework with oxidase and peroxidase mimicking activity for colorimetric detection of hydrogen peroxide. Microchim. Acta 184, 4629–4635 (2017)
T. Zhan, J. Kang, X. Li, L. Pan, G. Li, W. Hou, NiFe layered double hydroxide nanosheets as an efficiently mimic enzyme for colorimetric determination of glucose and H2O2. Sens. Actuator B-Chem 255, 2635–2642 (2017)
M. Chen, B. Yang, J. Zhu, H. Liu, X. Zhang, X. Zheng, FePt nanoparticles-decorated graphene oxide nanosheets as enhanced peroxidase mimics for sensitive response to H2O2. Mater. Sci. Eng. C. 90, 610–620 (2018)
R. Guo, Y. Wang, S. Yu, W. Zhu, F. Zheng, W. Liu, D. Zhang, J. Wang, Dual role of hydrogen peroxide on the oxidase-like activity of nanoceria and its application for colorimetric hydrogen peroxide and glucose sensing. RSC. Adv. 6, 577–583 (2016)
L. Chen, B. Sun, X. Wang, F. Qiao, S. Ai, 2D ultrathin nanosheets of Co–Al layered double hydroxides prepared in l-asparagine solution: enhanced peroxidase-like activity and colorimetric detection of glucose. J. Mater. Chem. B. 1, 2268–2274 (2013)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aghayan, M., Mahmoudi, A., Nazari, K. et al. Fe(III) porphyrin metal–organic framework as an artificial enzyme mimics and its application in biosensing of glucose and H2O2. J Porous Mater 26, 1507–1521 (2019). https://doi.org/10.1007/s10934-019-00748-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-019-00748-4