Abstract
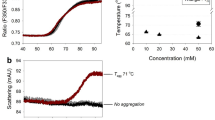
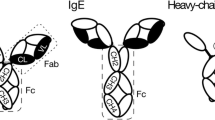
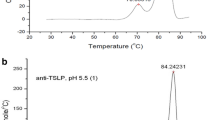
The purpose of this research is to study the thermal unfolding of high concentration bovine Immunoglobulin G (IgG) under 26 different experimental conditions by Fourier Transform Infrared spectroscopy with improved purge conditions and software calculations. When bovine IgG (25–200 mg/mL) was thermally denatured between pH 4.0 and 8.0, it was observed that at 25 mg/mL concentration, the protein exhibited maximum thermal stability at pH 6.0 and 7.0 as evident from the apparent Tm values. Increasing the concentration from 25 to 100 mg/mL at those pH values increased the thermal resistance of the protein by 2–3 °C. But, at 200 mg/mL, IgG showed a small decrease in its transition temperature. Presence of 100 mM Trehalose enhanced the Tm values at all conditions and possibly prevented the complete loss of IgG as insoluble aggregates at higher temperatures. Second derivative plots were constructed to explain the conformational changes of IgG during thermal unfolding.








Similar content being viewed by others
Abbreviations
- FTIR spectroscopy:
-
Fourier transform infrared spectroscopy
- Immunoglobulin G:
-
IgG
- CD:
-
Circular dichroism
- DSC:
-
Differential scanning calorimetry
- NMR:
-
Nuclear magnetic resonance
References
Davidson GP, Whyte PB, Daniels E, Franklin K, Nunan H, McCloud PI, Moore AG, Moore DJ (1989) Lancet 2:709–712
Devi VS, Chidi OO, Coleman D (2009) Spectroscopy 23:265–270
Dominguez E, Perez MD, Calvo M (1997) J Dairy Sci 80:3182–3187
Dominguez E, Perez MD, Puyol P, Sanchez L, Calvo M (2001) J Dairy Res 68:511–518
Dong A, Huang P, Caughey WS (1990) Biochemistry 29:3303–3308
Efron B, Tibshirani RJ (1993) In: Hall C (ed) An introduction to the bootstrap. Chapman and Hall/CRC Press, Boca Raton, pp 1–456
Gapper LW, Copestake DE, Otter DE, Indyk HE (2007) Anal Bioanal Chem 389:93–109
Gorga JC, Dong A, Manning MC, Woody RW, Caughey WS, Strominger JL (1989) Proc Natl Acad Sci USA 86:2321–2325
Guo J, Harn N, Robbins A, Dougherty R, Middaugh CR (2006) Biochemistry 45:8686–8696
Harn N, Allan C, Oliver C, Middaugh CR (2007) J Pharm Sci 96:532–546
Herron JN, Jiskoot W, Crommelin DJA (eds) (1995) Physical methods to characterize pharmaceutical proteins. Plenum Press, New York, pp 1–380
Ionescu RM, Vlasak J, Price C, Kirchmeier M (2008) J Pharm Sci 97:1414–1426
Jain NK, Roy I (2009) Protein Sci 18:24–36
Kaushik JK, Bhat R (2003) J Biol Chem 278:26458–26465
Korhonen H, Marnila P, Gill HS (2000) Br J Nutr 84(Suppl 1):S135–S146
Lee JC, Timasheff SN (1981) J Biol Chem 256:7193–7201
Li SQ, Bomser JA, Zhang QH (2005) J Agric Food Chem 53:663–670
Matheus S, Friess W, Mahler HC (2006) Pharm Res 23:1350–1363
Matheus S, Mahler HC, Friess W (2006) Pharm Res 23:1617–1627
Minton AP (2005) J Pharm Sci 94:1668–1675
Pelton JT, McLean LR (2000) Anal Biochem 277:167–176
Rahmelow K, Hubner W (1997) Appl Spectrosc 51:160–170
Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC (2005) Nat Biotechnol 23:1073–1078
Rosenberg AS (2006) AAPS J 8:E501–E507
Salnikova MS, Middaugh CR, Rytting JH (2008) Int J Pharm 358:108–113
Shire SJ, Shahrokh Z, Liu J (2004) J Pharm Sci 93:1390–1402
Tacket CO, Losonsky G, Link H, Hoang Y, Guesry P, Hilpert H, Levine MM (1988) N Engl J Med 318:1240–1243
Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC (1997) Nature 388:539–547
Tsioulpas A, Lewis MJ, Grandison AS (2007) Int J Dairy Technol 60:96–97
Venyaminov S, Prendergast FG (1997) Anal Biochem 248:234–245
Vermeer AW, Norde W (2000) Biophys J 78:394–404
Vermeer AW, Norde W, van Amerongen A (2000) Biophys J 79:2150–2154
Wang W, Singh S, Zeng DL, King K, Nema S (2007) J Pharm Sci 96:1–26
Wartewig S, Neubert RH (2005) Adv Drug Deliv Rev 57:1144–1170
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sathya Devi, V., Coleman, D.R. & Truntzer, J. Thermal Unfolding Curves of High Concentration Bovine IgG Measured by FTIR Spectroscopy. Protein J 30, 395–403 (2011). https://doi.org/10.1007/s10930-011-9344-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-011-9344-y