Abstract
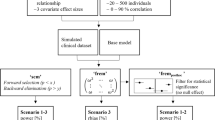
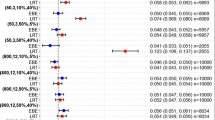
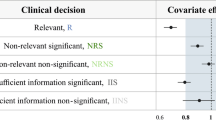
This study explored how study design influences the probability of selecting a ‘true’ covariate from two competing covariate models. The probability of selecting the ‘True Model’ (lean body weight on clearance) over the ‘False Model’ (total body weight (WT) on clearance) was compared for designs where WT was either lognormally distributed (i.e. non-stratified), or stratified into 3 equal strata. The probability of selecting the ‘True Model’ increased as the WT inclusion criterion widened, and was always greater under the stratified design. Incorporating stratification into study designs, in combination with a wide covariate range, can aid identification of true parameter–covariate relationships. This has particular importance if the model is to be extrapolated beyond the studied population (e.g. dosing in obesity).






Similar content being viewed by others
References
Miller R, Ewy W, Corrigan BW, Ouellet D, Hermann D, Kowalski KG et al (2005) How modeling and simulation have enhanced decision making in new drug development. J Pharmacokinet Pharmacodyn 32:185–197. doi:10.1007/s10928-005-0074-7
Food and Drug Administration (1999) Guidance for industry: population pharmacokinetics. U.S. Department of Health and Human Services, Rockville, MD
Gieschke R, Steimer J-L (2000) Pharmacometrics: modelling and simulation tools to improve decision making in clinical drug development. Eur J Drug Metab Pharmacokinet 25:49–58
Chien JY, Friedrich S, Heathman MA, de Alwis DP, Sinha V (2005) Pharmacokinetics/pharmacodynamics and the stages of drug development: role of modeling and simulation. AAPS J 7:E544–E559. doi:10.1208/aapsj070355
Lalonde RL, Kowalski KG, Hutmacher MM, Ewy W, Nichols DJ, Milligan PA et al (2007) Model-based drug development. Clin Pharmacol Ther 82:21–32. doi:10.1038/sj.clpt.6100235
Duffull SB, Waterhouse T, Eccleston J (2005) Some considerations on the design of population pharmacokinetic studies. J Pharmacokinet Pharmacodyn 32:441–457. doi:10.1007/s10928-005-0034-2
Holford NHG, Kimko HC, Monteleone JPR, Peck CC (2000) Simulation of clinical trials. Annu Rev Pharmacol Toxicol 40:209–234. doi:10.1146/annurev.pharmtox.40.1.209
Ribbing J, Hooker AC, Jonsson EN (2008) Non-bayesian knowledge propagation using model-based analysis of data from multiple clinical studies. J Pharmacokinet Pharmacodyn 35:117–137. doi:10.1007/s10928-007-9079-8
Maitre PO, Bührer M, Thomson D, Stanski DR (1991) A three-step approach combining Bayesian regression and NONMEM population analysis: application to midazolam. J Pharmacokinet Biopharm 19:377–384. doi:10.1007/BF01061662
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm 20:511–528. doi:10.1007/BF01061469
Jonsson EN, Karlsson MO (1998) Automated covariate model building within NONMEM. Pharm Res 15:1463–1468. doi:10.1023/A:1011970125687
Kowalski KG, Hutmacher MM (2001) Efficient screening of covariates in population models using Wald’s approximation to the likelihood ratio test. J Pharmacokinet Pharmacodyn 28:253–275. doi:10.1023/A:1011579109640
McLeay S, Morrish G, Kirkpatrick CMJ, Green B (2007) Lean bodyweight as a descriptor for propofol clearance. AAPS J 9:W4527
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387. doi:10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
Ribbing J, Jonsson EN (2004) Power, selection bias and predictive performance of the population pharmacokinetic covariate model. J Pharmacokinet Pharmacodyn 31:109–134. doi:10.1023/B:JOPA.0000034404.86036.72
Han PY, Duffull SB, Kirkpatrick CMJ, Green B (2007) Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther 82:505–508. doi:10.1038/sj.clpt.6100381
Duffull SB, Dooley MJ, Green B, Poole SG, Kirkpatrick CMJ (2004) A standard weight descriptor for dose adjustment in the obese patient. Clin Pharmacokinet 43:1167–1178. doi:10.2165/00003088-200443150-00007
Retout S, Mentré F, Bruno R (2002) Fisher information matrix for non-linear mixed-effects models: evaluation and application for optimal design of enoxaparin population pharmacokinetics. Stat Med 21:2623–2639. doi:10.1002/sim.1041
Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B (2005) Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065. doi:10.2165/00003088-200544100-00004
Ette EI, Williams PJ (2004) Population pharmacokinetics I: background, concepts and models. Ann Pharmacother 38:1702–1706. doi:10.1345/aph.1D374
Podranski T, Bouillon T, Schumacher PM, Taguchi A, Sessler DI, Kurz A (2005) Compartmental pharmacokinetics of dantrolene in adults: do malignant hyperthermia association dosing guidelines work? Anesth Analg 101:1695–1699. doi:10.1213/01.ANE.0000184184.40504.F3
Aarons L, Balant LP, Mentré F, Morselli PL, Rowland M, Steimer J-L et al (1996) Practical experience and issues in designing and performing population pharmacokinetic/pharmacodynamic studies. Eur J Clin Pharmacol 49:251–254. doi:10.1007/BF00226323
Food and Drug Administration (1998) Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labelling. U.S. Department of Health and Human Services, Rockville, MD
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20. doi:10.1038/sj.clpt.6100241
Karlsson MO, Holford NHG (2008) A tutorial on visual predictive checks. PAGE 17 Abstr 1434, www.page-meeting.org/?abstract=1434
Green B, Duffull SB (2003) Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol 56:96–103. doi:10.1046/j.1365-2125.2003.01849.x
Wählby U, Jonsson EN, Karlsson MO (2001) Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn 28:231–252. doi:10.1023/A:1011527125570
Acknowledgements
P.Y. Han was supported by a grant from Pfizer Global R&D. We would also like to acknowledge Stephen Duffull for valuable discussion and suggestions on this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, P.Y., Kirkpatrick, C.M.J. & Green, B. Informative study designs to identify true parameter–covariate relationships. J Pharmacokinet Pharmacodyn 36, 147–163 (2009). https://doi.org/10.1007/s10928-009-9115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-009-9115-y