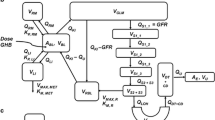
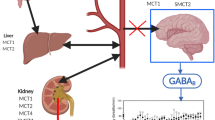
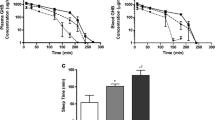
Recreational abuse or overdose of γ-hydroxybutyric acid (GHB) results in dose-dependent central nervous system (CNS) effects including death. As GHB undergoes monocarboxylic acid transporter (MCT)-mediated transport across the blood–brain barrier (BBB), one possible strategy for the management of GHB toxicity/overdose involves inhibition of GHB BBB transport. To test this strategy, interactions between GHB and MCT substrates (salicylic acid or probenecid) were simulated. Competitive, noncompetitive and uncompetitive inhibition mechanisms were incorporated into the GHB–MCT substrate interaction model for inhibitor dosing either pre-, concurrent or post-GHB administration. Simulations suggested that salicylic acid was the better candidate to limit GHB accumulation in the CNS. A time window of effect (> 10% change) was observed for salicylic acid pre- and post-administration, with maximal transport inhibition occurring within 12 hr of pre- and 2 hr of post-administration. Consistent with the prediction that reduced GHB brain concentrations could translate to decreased pharmacodynamic effects, a pilot study in rats showed that the pronounced GHB sedative/hypnotic effects (24.0 ± 6.51 min; n = 4) in the control group (1.58 mmol/kg GHB plus saline) were significantly (p < 0.05) abrogated by salicylic acid (1.25 mmol/kg) coadministration.
Similar content being viewed by others
References
Roth R.H. (1970). Formation and regional distribution of gamma-hydroxybutyric acid in mammalian brain. Biochem. Pharmacol. 19:3013–3019
Nelson T., Kaufman E., Kline J., Sokoloff L. (1981). The extraneural distribution of gamma-hydroxybutyrate. J. Neurochem. 37:1345–1348
Dyer J.E. (1991). Gamma-Hydroxybutyrate: a health-food product producing coma and seizurelike activity. Am. J. Emerg. Med. 9:321–324
Dyer J.E., Roth B., and Hyma B.A. (2001). Gamma-hydroxybutyrate withdrawal syndrome. Ann. Emerg. Med. 37:147–153
Okun M.S., Boothby L.A., Bartfield R.B., and Doering P.L. (2001). GHB: an important pharmacologic and clinical update. J. Pharm. Pharm. Sci. 4:167–175
Nicholson K.L., Balster R.L. (2001). GHB: a new and novel drug of abuse. Drug Alcohol Depend. 63:1–22
Lettieri J.T., Fung H.L. (1979). Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J. Pharmacol. Exp. Ther. 208:7–11
Saunders N.R., Habgood M.D., and Dziegielewska K.M. (1999). Barrier mechanisms in the brain, I. Adult brain. Clin. Exp. Pharmacol. Physiol. 26:11–19
Pardridge W.M. (1997). Drug delivery to the brain. J. Cereb. Blood Flow Metab. 17:713–731
Lee G., Dallas S., Hong M., and Bendayan R. (2001). Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol. Rev. 53:569–596
Bhattacharya I., Boje K.M. (2004). GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J. Pharmacol. Exp. Ther. 311:92–98
Echols R.M., Heyd A., O’Keeffe B.J., Schacht P. (1994). Single-dose ciprofloxacin for the treatment of uncomplicated gonorrhea: a worldwide summary. Sex. Transm. Dis. 21:345–352
Augenbraun M.H., and Rolfs R. (1999). Treatment of syphilis, 1998: nonpregnant adults. Clin. Infect. Dis. 28 Suppl 1:S21–28
Pao D., Goh B.T., and Bingham J.S. (2002). Management issues in syphilis. Drugs 62:1447–1461
Haverkos H.W. (1991). Infectious diseases and drug abuse. Prevention and treatment in the drug abuse treatment system. J. Subst. Abuse Treat. 8:269–275
Hwang L.Y., Ross M.W., Zack C., Bull L., Rickman K., and Holleman M. (2000). Prevalence of sexually transmitted infections and associated risk factors among populations of drug abusers. Clin. Infect. Dis. 31:920–926
M. Clinical Pharmacology. Valproic Acid http://cp.gsm.com/.
Wroblewski B.A., Joseph A.B., Kupfer J., and Kalliel K. (1997). Effectiveness of valproic acid on destructive and aggressive behaviours in patients with acquired brain injury. Brain Inj. 11:37–47
Rosenberg G. (1990). Brain Fluids and Metabolism. Oxford University Press, New York, pp. 15–53
Davies B., Morris T. (1993). Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093–1095
Coolens J.L., Van Baelen H., Heyns W. (1987). Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J. Steroid Biochem. 26:197–202
Gonzalez M.A., Tozer T.N., and Chang T.T. (1975). Nonlinear tissue disposition: salicylic acid in rat brain. J. Pharm. Sci. 64:99–103
Hirate J., Kato Y., Horikoshi I., Nagase S., and Ueda C.T. (1989). Further observations on the disposition characteristics of salicylic acid in analbuminemic rats. Biopharm. Drug Dispos. 10:299–309
Yue T.L., and Varma D.R. (1982). Pharmacokinetics, metabolism and disposition of salicylate in protein-deficient rats. Drug Metab. Dispos. 10:147–152
Emanuelsson B.M., and Paalzow L.K. (1988). Dose-dependent pharmacokinetics of probenecid in the rat. Biopharm. Drug Dispos. 9:59–70
Terasaki T., Takakuwa S., Moritani S., and Tsuji A. (1991). Transport of monocarboxylic acids at the blood-brain barrier: studies with monolayers of primary cultured bovine brain capillary endothelial cells. J. Pharmacol. Exp. Ther. 258:932–937
Kaufman E.E., Nelson T., Goochee C., and Sokoloff L. (1979). Purification and characterization of an NADP+-linked alcohol oxido-reductase which catalyzes the interconversion of gamma-hydroxybutyrate and succinic semialdehyde. J. Neurochem. 32:699–712
Kaufman E.E., and Nelson T. (1981). Kinetics of coupled gamma-hydroxybutyrate oxidation and D-glucuronate reduction by an NADP+-dependent oxidoreductase. J. Biol. Chem. 256:6890–6894
Giarman N.J., and Roth R.H. (1964). Differential estimation of gamma-butyrolactone and gamma-hydroxybutyric acid in rat blood and brain. Science 145:583–584
Perel J.M., Levitt M., and Dunner D.L. (1974). Plasma and cerebrospinal fluid probenecid concentrations as related to accumulation of acidic biogenic amine metabolites in man. Psychopharmacologia 35:83–90
Borgen L.A., Okerholm R.A., Lai A., and Scharf M.B. (2004). The pharmacokinetics of sodium oxybate oral solution following acute and chronic administration to narcoleptic patients. J. Clin. Pharmacol. 44:253–257
Kalasinsky K.S., Dixon M.M., Schmunk G.A., and Kish S.J. (2001). Blood, brain, and hair GHB concentrations following fatal ingestion. J. Forensic Sci. 46:728–730
Deguchi Y., Nozawa K., Yamada S., Yokoyama Y., and Kimura R. (1997). Quantitative evaluation of brain distribution and blood-brain barrier efflux transport of probenecid in rats by microdialysis: possible involvement of the monocarboxylic acid transport system. J. Pharmacol. Exp. Ther. 280:551–560
Dromgoole S.H., and Furst D.E. (1994). Salicylates. In: Evans W.E., Schentag J.J., and Jusko W.J. (eds) Applied Pharmacokinetics Principles of Therapeutic Drug Monitoring. Applied Therapeutics, Inc, Vancouver, Washington, pp. 32–34
Roth R., Giarman N. (1966). Gamma-butyrolactone and gamma-hydroxybutyric acid-I. Distribution and metabolism. Biochem. Pharmacol. 15:1333–1348
Enerson B.E., and Drewes L.R. (2003). Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J. Pharm. Sci. 92:1531–1544
Morris M.E., Hu K., and Wang Q. (2005). Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J. Pharmacol. Exp. Ther. 313:1194–1202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharya, I., Boje, K.M.K. Potential γ-Hydroxybutyric acid (GHB) Drug Interactions Through Blood–Brain Barrier Transport Inhibition: A Pharmacokinetic Simulation-Based Evaluation. J Pharmacokinet Pharmacodyn 33, 657–681 (2006). https://doi.org/10.1007/s10928-006-9029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-006-9029-x