Abstract
Immobilized microorganisms especially bacteria are most used rather than free cells to be protected from the environmental conditions when being used for the bioremediation of environmental pollutants. Herein, two marine’s bacterial isolates were tested for their ability to decompose crude oil. The optimum conditions for effective bacterial degradation e.g., pH, temperature, and inoculum size were investigated. PVA-alginate-clay composite hydrogel beads with different types of incorporated mineral clays were prepared and tested as bacterial carrier for potential bioremediation. Synthesized composite hydrogels were physico-chemically characterized by FTIR, SEM, and thermal analyses. Results showed that, embedded degrading bacteria in PVA-alginate beads recorded degradation rates as 74 and 66.6% for both tested bacterial isolates (S and R) compared to 61.2 and 53% degradation rates by free cells, respectively. Where, attapulgite clay-containing beads recorded maximum degradation% as 78.8 and 75% for both bacterial isolates, when added to immobilization matrices and these percentages could be enhanced under optimal conditions. The 16S rRNA gene of the two marine oils degrading bacterial isolates were amplified and sequenced, where both isolates were identified as Pseudomonas stutzeri and Rhodococcus qingshengii with submitted accession numbers of ON908963 and ON908962, respectively. These results are referring to the ability of using both tested isolates for crude oil bioremediation process and embedded them into PVA-alginate-clay beads as hydrogel carrier under the optimum conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Petrol related products, poly aromatic hydrocarbons (PAHs)), and pesticide residues, are currently the most hazardous contaminants represent a serious threat to the environment and the aquatic life and considered as a major challenge because of the advancement of industries and modernization. Crude oil contaminated sites such as soil and seas are a persistent problem with detrimental consequence because of the stubborn, toxic, and carcinogenic constituents of crude oil [1]. Bioremediation or biodegradation process is a waste treatment technology defined as the conversion of chemical pollutants into biomass, H2O and CO2 can be released by microorganisms [2], which is considered a low cost and effective solution [3]. It was proven that immobilizing microorganisms into polymeric matrices is more efficient than using free cells because the polymeric hydrogels have a lot of advantages allowing long term and effective biodegradation, in addition to protecting the microbes from the harsh environmental conditions [4].
The immobilization carriers are vital for successful bioremediation as they provide protection to the inoculated microbes, and they should be environmentally friendly with high affinity to the microorganisms and the PAHs [5]. Selection of bacterial supporting material is critical since the carrier can control the metabolic activity, maintain microbial stability and safeguards from a hostile and dangerous external environmental threats and hence improve the biodegradation percentages [6]. Specific criteria must be fulfilled in the chosen immobilization substrate such as having light weight, low cost, chemically inert, physically and chemically stable, non-polluting, nontoxic, aqueous insoluble and adapted to bacterial immobilization process [7].
PVA is a synthetic polyhydroxy polymer with high biocompatibility and mechanical qualities, PVA hydrogels are safe and clean rubber like materials, so, they have been widely used for drug delivery, tissue engineering, and other biomedical applications. PVA hydrogels are also known to be porous, so, they can be used as bacterial carriers [8] but have high degree of swelling ability in water, so, they must be chemically or physically cross linked to decrease their hydrophilic nature to be more stable bacterial carriers [9]. Alginate is a natural polysaccharide that extracted from brown algae [10]. It is characterized by its high binding affinity to calcium ions, it also can form biodegradable porous gel matrix, so, it is suitable as an inoculant carrier [11].
Mixed matrices of PVA and alginate have desired properties such as high mechanical stability and reusability, so, PVA- alginate beads showed better performance in PAHs, bioremediation compared to alginate beads [12]. PVA is incorporated in the PVA-alginate entrapping beads by intermolecular and intramolecular hydrogen bond between-OH groups [13]. Recent studies showed that the immobilized microbes have strong potentials to degrade environmental pollutants that their counterpart free cells [14], where tested the immobilization of microbes into powders made from nutshells, activated charcoal and organic materials in order to remediate crude oil-polluted soils. Moreover, in another study, the researchers reported the ability of Exiguobacterium sp. AO-11 strain immobilized into bio-cord carrier to effectively degrade crude oil of the contaminated environment with the efficiency of the immobilized cells to be reused for at least 5 cycles [15].
Different clay minerals were chosen as supportive materials due to their nontoxicity to bacterial cells, low cost and providing high surface area and mechanical stability, so, they can be used to reinforce the PVA-alginate beads and can be employed as a porous based material allowing the oxygen diffusion into the embedded microbe in the pores [16]. Also, the addition of a mineral clay acts as a physical crosslinker to improve the mechanical properties of the composite beads [17].
Attapulgite (ATP) is a natural hydrophilic clay mineral known for its reactive –OH groups on the surface and having a layer chain like structure with exchangeable cations in its framework channel. It was reported by Zhu et al. [18] that modified hydrophobic ATP through cation exchange showed high absorption capacity and selectivity to organic solvents and oils owing to its mesoporous structure and hydrophobic treatment which allow it to be effectively applied for crude oil biodegradation issues.
This study aims to the isolation of effective biodegrading bacterial isolates that can be immobilized in polymeric PVA-alginate beads for crude oil bioremediation purpose and studying the optimum conditions such as the effect of pH, temperature, inoculum size, incubation period, shaking/static conditions, crude oil and clay concentration to obtain the best degradation percentages of crude oil. Furthermore, following up the degradation rates of crude oil using gravimetric analysis and GC–MS investigation. Also, the preparation of PVA- alginate composite hydrogel beads as bacterial carrier and their instrumental characterization using FTIR, SEM and TGA were also investigated. Moreover, further investigations were concerned by studying the effect of using different types of clays such as Attapulgite, Montmorillonite, Bentonite, Laponite and Apatite on the enhancement of the crude oil bioremediation process and the mechanical and thermal stability of the hydrogel beads. Attapulgite had a great concern in the current study and has been tested in different concentrations due to its low cost and natural availability.
Materials and Methods
Materials
PVA (typically average Mw = 72,000 g/mol; 98.9% hydrolyzed) was obtained from Biochemica, Germany. Sodium alginate (SA) was purchased from DaeJung chemicals & metals, Korea. Methylene chloride was purchased from Fluka, Chemika. Calcium chloride (Fine GRD 90%) was purchased from Fisher Scientific (Fairlawn, NJ, USA).
Different types of clay were used, where Attapulgite was obtained from Northwestern desert of Borg El-Arab, Egypt, sodium Montmorillonite was obtained from Süd-Chemie AG, Germany. Pure bentonite and Laponite-RD clays were obtained from KREMER PIGMENTE GmbH, Aichstetten, Germany. Hydroxyapatite was obtained from Nanoinglobal-China, China. Na-MMT, ATP and Laponite RD were organically modified by a cation exchange with a quaternary alkyl ammonium salts as intercalating agents, other chemicals were used without further purification.
Culture Media
Luria Bertani (LB)
LB Broth was used for the cultivation of the bacterial strains and was composed of (g/l): Yeast extract; 5, peptone; 10, and NaCl; 10. Final pH (at 25 °C) was adjusted as 7.0 ± 0.2.
Bushnell-Hass (BH)
BH Broth was used as the basal medium for the crude oil degradation experiments and was composed of (g/l): Magnesium sulphate; 0.2, Calcium chloride; 0.02, Monopotassium phosphate; 1.0, Dipotassium phosphate; 1.0, Ammonium nitrate; 1.0and Ferric chloride; 0.05. Final pH (at 25 °C) was adjusted as 7.0 ± 0.2 using hydrochloric acid (0.01 M) and Sodium hydroxide (0.01 M).
Methods
Sample Collection
Two different chemically polluted sites in Mediterranean Sea named S (31.235772 N, 29.890912 E) and R (31.285874 N, 29.936795 E), Alexandria, Egypt were targeted for the collection of samples. The collected samples from contaminated sites were thought to have some bacterial strains with potent capacity to decompose PAHs under controlled and specific conditions. The sampling was performed according to [19] with some modifications. Each sample was taken under aseptic conditions using sterile 50 ml falcon tubes which by directly dipping the tubes into the water surfaces where both tubes were opened and closed beneath the water surfaces by 30 cm. One milliliter of each sample was inoculated into 50 ml LB broth in sterile falcon tube. All the tubes were incubated at 30 °C and 150 rpm for 24 h and were used for the bacterial isolation process.
Isolation and Purification of Crude Oil Degrading Bacterial Strains
The capacity of microbes to grow on mineral salt medium (BH Broth) supplemented with crude oil as a sole carbon source was investigated according to Mishra et al. [20] with minor modifications. Serial dilution for the previously collected and incubated samples was performed to obtain pure bacterial colonies. From the lowest dilutions, 50 µl were spread over BH agar plates supplemented with 100 µl surface spread-crude oil followed by incubation at 30 °C for 7 days.s until obvious growth of colonies was observed. The growing colonies were considered as presumptive crude oil degraders. Further purification of these colonies on LB agar was performed and the purified colonies were preserved in 4 °C for subsequent use.
Screening of Crude Oil Degrading Bacteria
The isolated bacteria that showed an ability to grow over the crude oil containing BH plates were tested for their ability to degrade the crude oil in liquid media according to Mishra et al. [20] with minor modifications. Firstly, 20 ml sterile BH broth was added to 50 ml sterile falcon tubes followed by the addition of 1% crude oil. Each tube was separately inoculated with 100 µl of LB-growing bacterial isolate (OD600 ~ 0.7). All tubes were incubated at 30 °C for 7 days. After incubation, the residual oil of each tube was gravimetrically investigated. The bacterial isolates that showed the lowest oil residues were selected for the rest of the work.
Gravimetric Determination of Oil Residues
The residues that were remaining after the biodegradation of the crude oil by each bacterial isolate were estimated according to the following method: 30 ml of methylene chloride were added to the whole content of each flask in a separating funnel. After well mixing for three minutes, two separated immiscible layers were formed. The lower layer that was composed of the solvent including the residual oil was received into a clean and previously weighed glass beaker. All beakers were heated at 60 °C for two days till complete evaporation of the solvent. After cooling, the beakers were then weighted again and the difference between the two weights of the empty and the oil-containing beakers was estimated.
The degradation percentage was calculated according to the following equation:
Where, Wc is weight of control sample (g) and Ws is weight of tested sample (g).
Optimization of Crude Oil Degradation Conditions by the Free and Immobilized Bacteria
Different parameters were tested to optimize the best conditions for oil degradation by the selected bacterial isolates.
Effect of pH Value
Different pH values from 3 to 11 were tested for their effect on the viability and crude oil biodegradation activity of the tested bacterial cells. A volume of 100 µl of each selected bacterial isolate, previously cultured in LB broth, were incubated with 10 ml sterile BH broth containing 50 µl of crude oil in sterile falcon tubes. The pH of each tube was formerly adjusted to the required pH value using 0.01 M NaOH and 0.01 M HCl. All the tubes were incubated at 30 °C and 150 rpm for 3 days. The remaining crude oil was gravimetrically determined using methylene chloride as mentioned earlier.
Effect of Different Incubation Temperatures
Microbes ability to use hydrocarbons of the crude oil as a carbon source is influenced by the surrounding temperature. Different temperatures (25, 30 and 35 °C) were tested to determine the biochemical behavior of the bacterial isolates towards crude oil biodegradation at the selected temperatures. Each 100 ml of sterile BH broth in 250 ml conical flasks were mixed with 1 ml crude oil and 1 ml of each bacterial isolate previously grown in LB broth and immobilized in the polymeric beads. In addition, un-inoculated control flasks were also prepared. Each flask was incubated separately at one of the previously mentioned temperatures at 150 rpm for 7 days. At the end of the experiment, the optimum temperature that resulted in the lowest crude oil residues was investigated.
Effect of Crude Oil Concentration
Different crude oil concentrations: 0.5, 1, 1.5, 2 and 2.5% were amended to 100 ml sterile BH broth followed by the inoculation of 1 ml of LB overnight culture of each bacterial isolate, in addition to un-inoculated control flasks. All flasks were incubated at 30 °C and 150 rpm for 28 days in a rotatory shaker, and then the residual oil was gravimetrically measured to determine the optimum concentration of oil that would be effectively degraded.
Effect of Shaking/Static Conditions
Depending on the examined bacteria, shaking of microbial cultures would increases the amount and distribution of the dissolved oxygen which might resulted in a negative or beneficial impact on the overall process. In this experiment, 100 ml of sterile BH broth were amended with 1 ml crude oil and 1 ml overnight culture of each bacterial isolate already grown in LB broth. All the flasks were submitted to static at 0 and/or shaking at 150 rpm for 7 days at 30 °C. After incubation, all flasks were removed from the incubators, and the crude oil residues of each flask were measured and compared to control flasks (un-inoculated with bacteria) to find out the best remediation condition. The residual oil content was determined using gravimetric analysis as mentioned before.
Effect of Different Incubation Periods
Time has a significant impact on the biodegradation process as it is directly affecting the bacterial growth and their subsequent breakdown of the inoculated carbon source. To evaluate the effect of incubation time on the bioremediation of crude oil, various incubation periods were tested from 0 to 28 days. At the beginning, 1 ml of each bacterial isolate were cultured in LB broth and immobilized in the polymeric beads. In 250 ml flasks, 100 ml of sterile BH broth were mixed with 1 ml of crude oil and the prepared beads followed by incubation of the flasks at 30 °C and 150 rpm with weekly sampling to determine the proper time for efficient crude oil-biodegradation. The residual crude oil of each treatment was gravimetrically determined and compared with control flasks that included crude oil mixed media without microbes.
Effect of Clay Incorporation into Beads Hydrogels
Different types of clay such as Attapulgite, Montmorillonite, Bentonite, Laponite and Apatite were tested as physical cross-linkers or nanofillers to enhance the mechanical and thermal stability of the PVA-alginate beads that have been used as bacterial immobilization matrices. In this assay, 100 ml of sterile BH broth were amended with 1 ml of bacterial cultures previously entrapped in PVA/alginate/Clay beads and 1 ml of the tested crude oil. The flasks were then incubated at 30 °C and 150 rpm for 28 days to determine the most mechanically and thermally stable beads that help for the achieving of the higher biodegradation percentage of the crude oil. The crude oil residues were gravimetrically determined in both experimental flasks and bacterial-free control flasks. Different concentrations of organically modified Attapulgite clay (ATP: 0.25, 0.5, 1, and 2%) were added into the polymeric beads to determine the optimum concentration that would result in the enhancement of the crude oil bioremediation assay. Attapulgite clay has been specifically selected for this experiment as it is one of the most abundant and low-cost mineral clays in Egypt’s deserts. It has also been tested to determine the ideal clay concentration that offer the highest bioremediation rate, mechanical, and thermal stability with adequate hydrogel porosity for sufficient interaction between imbedded bacteria and crude oil in the surrounding media.
Effect of Inoculum Size
To evaluate the inoculum size effect on the degradation percentage of the crude oil; different inoculum sizes of 0.5, 1, 1.5, 2, and 2.5% of the selected isolates were amended into 100 ml sterile BH broth followed by the addition of 1 ml crude oil. All flasks were incubated at 30 °C and 150 rpm for 28 days with interval sampling. After that, the residual crude oil of each flask was gravimetrically meas28ured. The optimum inoculum size that resulted in the effective bioremediation was determined.
Immobilization of Bacterial Isolates into PVA-Alginate/Clay Composite Beads Hydrogel
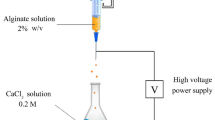
PVA-Alginate beads were prepared via external ionic gelation method [21, 22] by dissolving sodium alginate in distilled water (2% w/v) followed by co-dissolving of PVA (1% w/v) in the same solution at 80 °C. After complete solubility and clarity of the solution, 1% (w/v) Clay was added as a filler with continuous stirring to increase the homogeneity and then the polymeric solution was let to cool to the room temperature. After that, 1 ml of each selected bacterial culture that was previously grown in LB broth was separately added into10 ml of the prepared alginate/PVA/Clay solution under aseptic conditions. The solution was mixed well and was then dropped slowly through a syringe nozzle into 1% (w/v) CaCl2 solution as a cross linker. The solution was stirred gently using magnetic stirrer for at least 10 min as a curing time to enhance the mechanical strength. Similar procedures of using calcium chloride for crosslinking both alginate and PVA were reported by Baigorria et al. [23] and Narra et al. [24]. Different concentrations of PVA (0.5, 1, and 3% w/v), Alginate (1, 2, and 3% w/v), CaCl2 (0.5, 1, and 2%, w/v) and Attapulgite clay (0.25, 0.5, 1, and 2%, w/v) were tested.
Biodegradation of Crude Oil Using the Immobilized Bacteria
The whole amount of the immobilized bacteria that were entrapped inside the PVA/Alginate/Clay polymeric capsules were added to a 250 ml conical flask containing 100 ml of sterile BH broth mixed with 1 ml crude oil as a sole carbon and energy source. All the prepared flasks were then incubated in a rotatory shaker at 150 rpm and 30 °C. The degradation percentage was gravimetrically investigated every 7 days for a maximum 28 days successive.
Bioremediation of Crude Oil by Free and Immobilized Cells
It is thought that entrapped bacterial strains is more efficient than free cells for potential bioremediation process, because the encapsulation of bacteria in a polymeric matrix may offer rapid and complete degradation of the tested waste, as it provides bacterial protection from the environment, extreme pH and toxic materials that might existed in the contaminated sites, and also can provide higher biological stability of the tested strains [25]. To test that, 100 ml of sterile BH broth were incubated in 250 ml conical flasks with 1 ml of each free bacterial strains and 1% crude oil. At the same time, 1 ml of each tested bacterial isolate was entrapped in PVA/alginate and PVA/alginate/clay beads and were added to 100 ml sterile BH broth included 1% crude oil. After that, all flasks were incubated at 30 °C and 150 rpm for 28 days. Oil residues were measured gravimetrically, and the efficiency of the free and immobilized strains were compared to determine the best bioremediation condition.
Molecular Identification of Bacterial Isolates
-
Genomic DNA extraction
Total genomic DNA from each bacterial culture was extracted using Amshag kit according to the manual instructions of (SRTA-City, Egypt).
-
PCR amplification and sequencing of the 16S rRNA gene
Each extracted genomic DNA was used as a template for the amplification of 16S rRNA, through PCR technique. A multiplex PCR kit (Qiagen, USA) was used for the amplification of the target gene using universal primers. The forward (5`-GAA CGC GAA CCT TAC-3`) and reverse primers (5`-CGG TGT GTA CAA GGC CCG GGA ACG-3`) were used to amplify 500 bp of the 16S rRNA gene of each bacterial isolate. The reaction program was started with a first denaturation step at 94 °C for 4 min followed by 35 cycles of 30 s at 94 °C, 30 s. at 55 °C, and 30 s at 72 °C followed by a final extension step at 72 °C for 7 min. The amplified genes were submitted for electrophoresis in 1 × TBE buffer at 120 V for 30 min. The amplified genes were visualized and detected using Gel documentation system, compared with 1 kb DNA ladder. The obtained genes were subsequently submitted for purification and sequencing (SIGMA, Germany), and the obtained sequences were submitted in GenBank using new accession numbers. The phylogenetic tree of the obtained sequences and other related sequences in GenBank was performed using MEGA 11.0 program.
Swelling Behavior of PVA/SA Hydrogel Beads
The swelling ability of a hydrogel reflects its permeability and represents an important parameter for hydrogel performance and for immobilization process to ensure exchange action between the immobilized bacteria and the surrounding media. In this experiment, 1 g of dried PVA/SA beads (Wd) was soaked in distilled water at room temperature with interval sampling and weighting of the swelled beads (Ws) till reaching equilibrium [26]. Water uptake percentage was measured according to the following equation:
where Wd refers to the weight of the dry sample and Ws refers to the weight of the swollen sample at specific time intervals.
Instrumental characterization
FT-IR
Vacuumed and dried samples of pure PVA, sodium alginate, and PVA–Alginate hydrogel beads were examined by FTIR on an EQUINOX 55 instrument (BRUKER, Germany). The dry samples were ground together with infrared grade KBr and then pressed to make Translucent KBr-disks. The FTIR spectra were produced by recording 64 scans with a resolution of 2 cm−1 between 4000 and 400 cm−1. All samples were freeze-dried using liquid nitrogen, crushed to a fine powder (KBr: sample = 140 mg: 2 mg), and pressed into a clear transparent disk with a diameter of 13 mm by applying a force of 105 N.
Scanning Electron Microscopy
Microstructure morphology and surface characterization of immobilized S and R isolates in PVA-SA beads with/without Clay were performed after beads lyophilization using Analytical-SEM (type: JEOL, JSM-6360LA, Japan) with 15 kV voltage for secondary electron imaging. The hydrogel beads were dehydrated by freeze-dryer and coated with Au using an ion sputter coater (model: 11,430, USA, combined with vacuum base unit or SPi module control, model:11,425, USA).
Thermal Analysis
TGA thermograms were used to characterize the thermal properties of a vacuumed dried PVA/SA hydrogel beads using different clay concentrations (0, 0.25, 0.5, 1 and 2%). The thermo-gravimetric analysis (TGA) was carried out on a 204 Phoenix TGA instrument (NETZSCH, Germany) at a heating rate of 10 °C/min from room temperature to 600 °C. The onset temperature Tonset was calculated using TGA thermogram which are defined as the temperature at the point of inflection or highest mass loss percentage at the intersection of the baseline mass and the tangent drawn to the mass curve.
GC–MS Analysis
Crude oil and its metabolites after 28 days of biodegradation were analyzed using GC–MS technique compared with control sample. The GC–MS test was carried out using a Trace 1300 GC Ultra/Mass Spectrophotometer ISQ QD (Thermo Scientific) instrument, X-calibur 2.2 software (Thermo X-calibur) equipped with a TG-5MS Zebron capillary column (length 30 m × 0.25 mm ID, 0.25 µm film thickness; Thermo). Helium (average velocity 39 cm/s) was used as the carrier gas. The oven temperature program was: Held at 80 °C for 5 min then increased from 80 to 200 °C for 1 min (4 °C/min), 200–300 °C for 1 min (7 °C/min) and injector temperature 300 °C. Sample size was 1 µl; injection split ratio was 20:1 and run time was 51.29 min. Data analysis was carried out using NIST database.
Statistical Analysis
All the statistical analysis was performed using IBM SPSS® software, 1997, according to Duncan’s multiple range test [27].
It could be concluded that these methodologies targeting the proper immobilization of the tested bacterial cells inside the polymeric materials supported by clay component in order to enhance the crude oil bioremediation process under tested optimized conditions.
Results and Discussions
Isolation and Purification of Crude Oil Degrading Bacterial Strains
Contaminated samples that were collected from the Mediterranean Sea, Alexandria, Egypt were used for the isolation of microorganisms with crude oil degrading capacity. In this case, two bacterial isolates identified as Pseudomonas stutzeri and Rhodococcus qingshengii (S and R) were used as bacterial isolates from different locations that have been tested for their ability to degrade crude oil as a sole carbon and energy source. The obtained results revealed the ability of both bacterial isolates to degrade crude oil effectively. However, all other 8 bacterial isolates failed to show any signs of crude oil biodegradation ability, and hence all of them were ignored. On the other hand, both selected samples were then tested for their ability to degrade the tested crude oil under different optimization conditions as will be shown in the following experiments. Each experiment was repeated 2 or 3 times.
One Variable at Time Optimization (OVAT)
Effect of pH Value
Both crude oil degrading isolates were investigated for their bioremediation capacity at wide pH ranges as described in Fig. 1a and b. It has been shown that, both S and R isolates were able to grow in wide range of pH values including neutral, slightly alkaline, and slightly acidic medium ranging from 6 to 8 as the highest values resulted in higher biodegradation percentages. In both isolates, 50% degradation was recorded at slight acidity (6) and slight alkalinity (8). This percentage was gradually decreased to reach 33.3% at pH 4, 5 and 9 for isolate S; and 5 and 9 for isolate R, with lower degradation percentages at stronger acidic or alkaline conditions. However, the highest degradation percentage was recorded as 66.6% at neutral pH value (7) for both isolates.
Bacterial degradation in wide pH ranges is a good sign for the bacterial stability even under varying pH conditions or in different environments. Our results are almost matched with Simarro et al. [28], who showed that the desired pH for PAHs (Naphthalene, Phenanthrene and Anthracene) degradation ranging from 5.5 to 7.8 using Enterobacter Pseudomonas and Stenotrophomonas bacterial consortium, On contrary Das et al. [29] observed that the bacterial growth increased in alkaline medium at pH 8.5 in the degradation of P-Nitrophenol using potent Pseudomonas strain from the textile dye industry effluent. The previous results indicate that, pH regulates microbial enzyme activity, transport process and nutrient solubility, and so governs the microbial activity to great extent [30, 31].
Effect of Different Incubation Temperatures
The effect of temperature on the growth of both tested bacterial isolates at the presence of crude oil as a sole carbon and energy source was investigated. As shown in Fig. 1c and d, the optimum temperature was 30 °C recording 80 and 76.5 degradation percentage for both S and R isolates, respectively. This result consistent with the results obtained by Paliulis et al. [32] who showed that 30 °C is the optimum temperature for the highest oil hydrocarbon degradation by the use of Acinetobacter sp, and our results are also matched with Huang et al. [33] in selenite bioremediation using the highly selenite-tolerant strain (Providencia rettgeri HF16-A). Also, 35 °C showed high acceptable bioremediation results while moderate growth was found at 25 °C. These results indicate the wide range application of the tested Sand R isolates in different ranges of temperatures as crude oil degraders. Extreme temperatures (either too low or too high) have an impact on microbial growth and microbial enzyme-catalyzed processes. When the temperature rises with a reasonable range, the rate of bioremediation increases as microbial metabolism rises, but to a certain limit that can be bear with organisms. Moreover, the solubility of PAHs is also increasing at higher temperature, as a result, bioavailability increases [34].
Effect of Crude Oil Concentration
The ability of S and R isolates to mineralize different crude oil concentrations was tested as shown in Fig. 2a and b. Both showed a high degradation capacity at low crude oil concentrations. However, their ability to degrade crude oil was gradually reduced with increasing the oil concentrations. Maximum degradation rates of 85 and 83.5% were recorded at 0.5% crude oil concentration for S and R isolates, respectively. At 1% crude oil concentration, S and R isolates recorded 78.8 and 76.66% degradation rate, respectively. At 1.5% crude oil concentrations, 74.1 and 71.6% degradation rate were recorded for S and R isolates, respectively. Moderate degradation percentages of 71.6 and 69.6% were recorded at 2% crude oil, while 66.4 and 65% were recorded when 2.5% crude oil was used for S and R isolates, respectively. Our results are almost matched with Taha et al. [35] who showed that the degradation percentage decreased with increasing phenanthrene concentration using phenanthrene degrading bacteria (Enterobacter cloacae, Bacillus sp. and Bacillus thuringiensis) isolated from petroleum contaminated soil. Also, these results are consistent with the results obtained by Abtahi et al. [36] where the crude oil removal efficiency was decreased by increasing petroleum initial concentration in their study of the effect of competition between petroleum-degrading bacteria and indigenous compost microorganisms on the efficiency of petroleum sludge bioremediation using Acinetobacter radioresistens strain KA5 and Enterobacter hormaechei strain KA6 isolated from the petroleum waste sludge, as high levels of petroleum compounds are poisonous to the bacteria so, they preferred 1and 2% crude oil as optimum concentrations. Also, El-Noubi et al. [37] reported that fast bacterial growth occurred at low oil concentrations while the growth rate decreased and suppressed at high oil concentration so, they chose 0.6% crude oil concentration as optimum concentration. In the same context, Hazim et al. [38] reported that decreasing the microbial degradation activity at high hydrocarbon concentrations that can inhibit microorganisms through toxic effects, resulting in a decrease in biodegradation percentage in their study of the effect of petroleum hydrocarbons contamination with Kerosene, Diesel and lubricate oil on soil microorganisms and biodegradation. Moreover, Abarian et al. [39] observed that, the number of bacterial cells reduced by increasing phenol concentration in their study of degradation of phenol at high concentrations using immobilization of Pseudomonas putida P53 into sawdust entrapped in sodium-alginate beads. So, low, and moderate crude oil concentration was investigated as the most preferred concentrations for effective bioremediation using our tested isolates (S and R). Furthermore, Ibrahim et al. [40] demonstrated that 1% crude oil concentration was the optimum concentration as the sole source of carbon and energy in their research of crude oil degradation, and heavy metal tolerance. From these results, we can conclude that 0.5 and 1% crude oil can be considered as the optimum concentration in our experiments.
Effect of Shaking/Static Conditions
Bacterial growth significantly affected by shaking conditions which may subsequently affect the bioremediation process. Figures 2c and d show moderate degradation capability for both S and R isolates under static condition. The maximum crude oil degrading percentages were recorded when the microbes were shaken at 150 rpm. At this shaking rate, both of S and R isolates recorded degradation percentages of 76.7 and 70.6%, respectively. While moderate degradation rate of 61.2 and 58.8% were recorded using static conditions. These results were attributed to the crude oil dissolution and oxygen availability under shaking conditions which in turn enhanced the tested crude oil degradation rate. These results are consistent with A. Sed et al. [41] who explored the biodegradation of phenanthrene by Klebsiella sp isolated from organic contaminated sediment. Both S and R isolates showed degradation activity using 150 rpm shaking condition when crude oil was amended as a sole carbon and energy source. Accordingly, moderate shaking at 150 rpm was preferred as optimum condition than static condition for effective bioremediation in the current experiment.
Effect of Different Incubation Periods
By monitoring the crude oil degradation rates along 28 days as represented in Fig. 3a and b; results showed that, by increasing the incubation period from 7 to 28 days; the crude oil degradation percentages increased significantly. After 7 days of incubation, S and R isolates recorded 64.7 and 60% degradation rates, respectively. By increasing the incubation period to 14 days; the crude oil degradation rates increased to 68.2 and 64.7% for S and R isolates, respectively. Further increase in the degradation rates to 70.5 and 67% was recorded with increasing incubation period to 21 days for both S and R isolates, respectively. Maximum crude oil degradation rates of 76.66 and 70.5% were recorded after 28 days of incubation for S and R isolates, respectively, which indicates that 28 days of incubation are the optimum incubation periods for the tested bacteria. In the same context, Baoune et al. [42] reported that, after 28 days of incubation, significant decrease in crude oil concentration was observed compared to control flasks in petroleum hydrocarbons degradation in their study of bioremediation of crude oil-contaminated soils using Streptomyces sp. Hlh1. Also, our results matched with El-Sheshtawy et al. [43] who demonstrated that, the biodegradation percentage increased with increasing the incubation time in the degradation of petroleum hydrocarbons using Flavobacterium johnsoniae and Shewanella baltica bacterial strains immobilized on goethite-chitosan nanocomposite. Moreover, Usmani et al. [44] reported that the degradation rate of lindane pesticide was increased with the number of days of incubation in their study of bioremediation of lindane contaminated soil and exploring the potential of actinobacterial strains.
Effect of Clay Addition
Due to their high surface area, clays are commonly utilized as adsorbents. In their natural form, clays have low sorption capacity to hydrophobic organic compounds in aqueous solutions because their exchangeable ions are highly hydrated. Ionic exchange of their inorganic ions with cation makes the clay surface hydrophobic and in turn increases clays adsorption capacities towards hydrophobic compounds [45]. In this case, clays can be used in cleaning up hydrocarbon spills on the shoreline and land [46]. The effect of different types of mineral clays such as: organically modified Attapulgite, Laponite RD, hydroxyapatite, pure Sodium Montmorillonite, and pure Bentonite, on the degradation percentages of crude oil can be shown in Fig. 3c and d. It was found that, the maximum degradation rates of 85.9 and 84.7% for S and R isolates, respectively were recorded when apatite was added to the immobilization polymers. Second higher degradation rates were recorded when Laponite was used where it showed 82.4 and 78.33% for S and R isolates, respectively. While at the presence of Attapulgite, the degradation percentages were moderate and showed 78.8 and 75% for S and R isolates, respectively. Also, when Montmorillonite was incorporated, acceptable degradation rates were recorded as 70.5 and 68% for S and R isolates, respectively. On the other hand, the lowest degradation rates were recorded through the presence of Bentonite. The degradation percentages of 65.2 and 64.7% for S and R isolates; respectively, were recorded at its presence. These results may be attributed to the modification of the tested mineral clays by ion exchange using intercalating agents which increased their hydrophobic character and in turn enhance the interaction between the PVA- alginate beads and the hydrophobic crude oil in the surrounding media. These interactions resulted in a significant increase in the degradation rates that leading to more efficient degradation process. This finding of the research is novel as it shed light on the effect of different types of clays specially organically modified clay such as hydroxyapatite, laponite RD and attapulgite due to their higher sensitivity to hydrophobic crude oil compared to hydrophilic natural clays such as bentonite and montmorillonite making them a great addition into the hydrogel beads composition and providing higher bioremediation rates of the tested crude oil. A laboratory representative figure of the crude oil before and after biodegradation by the bacterial isolates at the presence of different clay types can be seen in Fig. S1 (supplementary data).
Moreover, the suggested mechanism for the effectiveness of bioremediation process through the addition of clay could be summarized as follows: The clay minerals are known as the most abundant minerals originated through the water–rock interactions. They have a lot of advantageous such as having surface charge, having large surface areas, and interlayer spaces, which allows them to effectively adsorb different types of polar and non-polar organic substances [47]; and hence find their way in thousands of industrial applications, environmental protection technologies, and remediation purposes [48, 49]. The colloidal sized clays have been used for the remediation of hydrocarbon contaminated solutions as they able to aid dispersion, and their large surface areas can positively accelerate the physical and chemical disaggregation processes in addition to the size reduction of the oil globules [50]. In addition, they potentially enhance the biodegradation of hydrocarbon compounds and increasing the rate of bacterial growth. This potentiality is linked to their ability adsorb the protons released during the breakdown of the hydrocarbons which plays an important role in avoiding the change in the optimal pH conditions, and hence sustaining the bacterial growth. Moreover, some clays able to stimulate the bacterial activity through the nutrient adsorption on the bacteria cell walls by the formation of C–O–Na–Si complexes on the outer surfaces of the bacterial cell walls which is associated with the particle dissolution. This is highly matched with [47], who reported that the addition of clay minerals with large specific surface areas and high cation exchange capacities are extremely improving the biodegradation of hydrocarbons by bacterial cells over a significant time period.
Effect of the Attapulgite Mineral Clay Concentration on the Crude Oil Degradation Rate
The effect of the concentration of the organically modified Attapulgite clay mineral (ATP) (extracted and purified by Elbassyoni et al. [51] from (Northwestern desert of Borg El-Arab, Egypt) on the bioremediation of crude oil was investigated as shown in Fig. 4a and b. When the concentration of ATP was 0.25%, the crude oil degradation rate reached its maximum recording 74.1and 73% for S and R isolates, respectively, and was gradually decreases by increasing the clay concentration. This indicates that the higher clay concentration in the prepared beads has a little and weak effect on the bioremediation process. These higher concentrations might block the pores of the beads that immobilizing the bacteria, which in turn inhibit the metabolic activity exchange between the bacteria and the outside medium and negatively affecting the degradation process. As a result, the addition of low clay concentrations to the beads could enhance their mechanical stability with adequate permeability and make the immobilized cells more efficient. Our results are almost matched with Ruan et al. [52] in their degradation of phenol using immobilized Sphingomonas sp. in PVA-alginate- kaolin beads as they reported that high kaolin content more than 1% in the hydrogel beads restricted the cells' ability to develop, reducing the activity of the bacteria and hence, decreasing the bioremediation rates. In the current investigation, 0.25% ATP was the optimal concentration in terms of degradation rate, mechanical strength, and cost making attapulgite clay a novel candidate in our study providing higher bioremediation percentages even in very low concentration. In the same context, Lin et al. [53] reported similar results in the degradation of TNT using immobilized Bacillus mycoides in PVA-alginate- kaolin beads as they demonstrated that lower kaolin concentration was preferred and the mechanical strength of the beads was much poorer without kaolin. Also, it increased the adsorption capacity of the beads and making the bacteria more active.
So, it could be concluded that it positively affected the biodegradation process.
Effect of Inoculum Size
Initial inoculum size is an important factor in bioremediation processes. Results showed that the bioremediation of crude oil was also inoculum size dependent. To evaluate the effect of inoculum size as a critical parameter on crude oil degradation rate, both S and R isolates were tested in different concentrations as shown in Fig. 4c and d. It was observed that, the degradation percentage decreases significantly by increasing inoculum size. It has been detected that, at 0.5% inoculum size, maximum degradation rates of 87.1 and 88.33% for S and R bacteria, respectively were recorded. At 1% inoculum size, the degradation rates recorded 80 and 83.5% for S and R isolates, respectively. Acceptable degradation rates of 74.1 and 64.7% for S and R bacteria, respectively, were recorded when 1.5% inoculum size was tested. Further decreases in the degradation rates were measured at 2% inoculum size and resulted in 71.8 and 61.2% degradation percentages for S and R isolates, respectively. The lowest degradation rates were recorded at 2.5% inoculum size and showed 69.4 and 57.65% for both S and R bacteria, respectively. Accordingly, 0.5% inoculum size was the optimum and preferred concentration in terms of degradation rates and cost. In the same context, Costa et al. [54] demonstrated that, higher inoculum concentration did not improve the biomass production, so, lower inoculum size was preferred (25 µl) in their study to use the glycerol from biodiesel production as a carbon source for biomass production by actinobacteria with well-known bioremediation abilities. In the current investigation, the results may be attributed to the depletion of the carbon source (petroleum oil components) along 28 days in addition to the releasing of poisonous metabolic products that might affect the activity of the bacterial cells. However, the opposite has been proved in other studies such as Philip et al. [55] who observed that, the increase in bacterial cell concentrations would increase the endosulfan degradation efficiency in their study using Staphylococcus sp., Bacillus circulans enriched from contaminated soil collected from the vicinity of an endosulfan processing industry.
Bioremediation of Crude Oil by Free and Immobilized Cells
Comparing the crude oil degradation rates by free and immobilized cells in PVA-alginate beads is represented in Fig. 5a and b. The maximum degradation percentages were recorded by immobilized bacterial isolates in the presence of ATP clay as nano-filler as 74 and 66.6% for S and R bacterial isolates, respectively. While 68.24 and 55.5% were recorded in the absence of clay by S and R isolates, respectively. The lowest degradation percentages of 61.2 and 53% were recorded by the free cells of S and R bacteria, respectively. From the previous results we can conclude that, immobilized bacterial cells acted more efficient than free ones. These results may be attributed to the large surface area offered by the beads which significantly increases the contact between the microbes and the crude oil under protection conditions, and hence resulted in high crude oil degradation percentages even at much higher oil concentrations. In the same context, Gouda et al. [56] observed that, the degradation rate of kerosine increased and the time of degradation reduced by immobilizing Gordonia sp. and Pseudomonas sp. in rice straw compared to free cells in their case study of bioremediation of kerosene in liquid media. These results were also matched with Talha et al. [57] in the bioremediation of Congo red dye using a free cell and cell immobilized on biochar of coconut shell. Moreover, Padmanaban et al. [58] reported that, immobilized cell systems provide more advantages over free cell systems in their study of degradation of reactive red 120 dye in bed reactor by bacillus cohnii RAPT1 immobilized on poly urethane foam (PUF). Additionally, Bayat et al. [59] demonstrated that the trapping of Pseudomonas aeruginosa UG14 in clay, alginate and skim milk for phenanthrene degradation along 30 days protected bacterial cells survival compared to free cells that lasted for only 18 days.
Bacterial Identification Using 16S rRNA Gene
The molecular identification of the bacterial isolates was depending on genomic DNA extraction, PCR amplification and sequencing of the 16S rRNA gene. The obtained results showed the successful amplification of 500 bp of the target gene for both bacterial isolates. After purification and sequencing of the amplified genes, the obtained sequences were submitted in GenBank to have new accession numbers. The isolate R was identified as Rhodococcus qingshengii and was submitted in GenBank with the accession number ON908962. While the isolate S was identified as Pseudomonas stutzeri and was submitted in GenBank with the accession number ON908963. The phylogenetic tree of the two isolates compared with other similar sequences deposited in GenBank as is illustrated in Graph 1.
Swelling Behavior of Hydrogel Beads
Swelling capacity is one of the undeniable assays required for bioremediation process to ensure exchange between the embedded bacteria inside the hydrogel beads and the surrounding environment. So, adequate degree of water uptake is required to facilitate the exchanging process. PVA-alginate beads with different PVA concentrations were immersed in distilled water to test their water uptake abilities as represented in Fig. 6a. It was practically observed that, the weight of the beads significantly increased with increasing the PVA content in the tested hydrogel beads which may be attributed to the higher hydrophilic nature of the PVA. In other words, higher PVA ratio resulted in higher swelling capacity due to increasing the number of –OH groups of the PVA compound, and hence making the beads more hydrophilic [60].
So, we can conclude that, there is an inverse relationship between swelling capacity and mechanical stability of the hydrogel beads, 0.5% PVA has lower swelling capacity with higher mechanical stability while 3% PVA has higher water uptake capacity due to increasing hydrophilicity but lower mechanical stability. So, 1% PVA was considered as the optimal concentration in our study as it provides moderate and adequate swelling capacity of the hydrogel beads and hence acceptable mechanical stability.
By following up the swelling ratios of the PVA-alginate composite beads with different alginate concentrations as represented in Fig. 6b; it was observed that increasing alginate content significantly depressed the swelling capacities of the tested beads. This may be attributed to the higher network density of the alginate hydrogels which prevents the swelling of the alginate chains in water as previously reported by Omidian et al. [61] that higher crosslinking density in alginate chains prevents them from expanding in water in their study of elastic, super porous hydrogel hybrids of polyacrylamide and sodium Alginate.
1 wt% alginate has higher swelling capacity and hence lower mechanical stability while 3 wt% alginate has lower water uptake and higher mechanical strength but condenser hydrogel network and hence lower exchange capacity between the embedded bacteria and the surrounding media. So, 2 wt% alginate was considered as the optimal concentration for acceptable exchange capacity and mechanical stability.
The blending of Alginate with other polymers such as polyvinyl alcohol (PVA) has been proposed to overcome the quick breakdown of hydrated alginate beads This is also consisting with Narra et al. [24] in their study of Rifampicin Loading on PVA-alginate beads by ionotropic gelation method. In addition, it is also proved that physical crosslinking via intra molecular hydrogen bonding (entanglement) occurs between the same PVA molecules and intermolecular hydrogen bonding between PVA and alginate chains by Baigorria et al. [23] in their study of arsenic removal.
CaCl2 concentration is another important factor that influences the swelling rate of the alginate beads as Ca2+ ions act as an ionic cross linker which leading to the solidification process of the alginate beads. As represented in Fig. 6c, it was observed that increasing Ca2+ concentration up to 2 wt% significantly decreased the swelling capacity of the tested composite beads because of increasing the crosslinking density and hence decreasing water uptake ratios. 2 wt% CaCl2 has lower swelling capacity and higher mechanical stability due to higher crosslinking degree while 0.5 wt% CaCl2 has higher swelling ratio and in turn lower mechanical stability because of lower crosslinking density and then faster rapture of the hydrogel beads. So, 1 wt% CaCl2 was considered as optimal concentration to get suitable exchange capacity required for the bioremediation process, the same results were reported by Nunes et al. [62] who reported that, cross-linked polymer networks can be characterized by swelling measurements, which are useful for understanding drug release and diffusion transport processes via macromolecular materials. By monitoring the effect of ATP clay on the swelling behavior of the clay composite hydrogel beads; it was observed that, the swelling ratios decreased by increasing the clay concentration as represented in Fig. 6d. These results can be explained as the incorporation of ATP clay to the composite hydrogels act as an additional physical cross linker leading to more condenser network and hence decreasing the water uptake. The same results were demonstrated by Golafshan et al. [63] who found that laponite concentrations higher than 0.5% significantly reduced capacity of hydrogel beads to swell during their study of nano hybrid hydrogels of PVA-Alginate-Laponite as a potential wound healing material. So, 0.25% clay was considered as optimum concentration in our study as it also provides higher degradation rate as previously reported.
FTIR Analysis
The FTIR spectra of pure Alginate, PVA, and CaCl2 crosslinked PVA-alginate beads are represented in Fig. 7. The spectra associated with alginate showed that, Symmetric and a symmetric stretching band of assigned to carboxylate groups appeared at 1406 and 1612 cm−1, respectively. Moreover, the appeared peak at 1022 cm−1 is related to C–O–C stretching vibration [64]. A characteristic broad strong band assigned to hydroxyl group stretching vibration appeared at 3200–3600 cm−1 and the peak at 2987 cm−1 is corresponding to C–H stretching [65].
The FTIR spectra associated with pure PVA showed that, a broad band at 2887 cm−1 represents C–H stretching vibration of the alkyl groups of the PVA [59] while carboxyl (C–O) stretching band appeared approximately at 1107 cm−1. The peak at 1466 cm−1 represents the C-H bending, while the band at 1718 cm−1 assigned from C=O stretching vibration [66].
The spectrum of PVA-SA beads shows abroad absorption band in the region of 3200 to 3600 cm−1 which is related to stretching vibration of –OH groups of both PVA and alginate [23]. Moreover, absorption bands at 1600 and 1410 cm−1 represents asymmetric and symmetric stretching vibrations of –COO groups [21]. The band recorded at 1248 cm−1 may be related to H–bonding between –O–H groups of alginates and PVA. A strong absorption peak that recorded at 1066 cm−1 belongs to –C–O stretching [67]. Two peaks were determined at 2980 and 2906 cm−1 which attributed to –C–H stretching vibration. The incorporation of ATP clay doesn’t chemically affect the polymer matrix as it acts as physical filler.
SEM Investigation
The micrographs of PVA-alginate composite hydrogel beads were investigated using SEM micrographs as are represented in Fig. 8. The surface morphology of the dry beads is represented in Fig. 8a shows smooth surface that indicating crosslinking reaction between polymers chains with tiny pores in nano size. Figure 8b represents porous network of lyophilized wet PVA-alginate beads before bacterial immobilization, which has a similar morphology as observed by Shivakumara et al. [65]. However, the immobilized bacterial cells showed irregular and rough surface indicating wide distribution of bacterial cells inside the alginate composite polymeric matrix and the proper immobilization process as represented in Fig. 8c and d. SEM images of the clay-containing hydrogel beads as shown in Fig. 8e and f, confirmed the distribution of the ATP clay in the PVA-alginate matrix with improving the hydrogel physical properties (such as the mechanical and thermal stability) because of sufficient interaction between the ATP clay as a physical crosslinker and the polymer matrix. Similar results were previously determined by Golafshan et al. [63].
Thermal Gravimetric Analysis
TGA analysis was used to investigate the effect of ATP clay on the thermal decomposition of PVA-alginate composite hydrogel beads. TGA curves of dried PVA-alginate beads with different ATP content (0, 0.25, 0.5, 1 and 2 wt%) are represented in Fig. 9 and summarized in Table 1. The thermal decomposition of PVA- alginate hydrogels occur in three major steps. The first decomposition stage or dehydration stage between 100 and 200 °C is due to the evaporation of water and stored humidity. The second decomposition stage or de-polymerization stage between 200 and 380 °C is related to the PVA elimination reactions and the cleavage of the main chains of alginate [68].The third stage or pyrolysis stage is between 380 and 600 °C where NaHCO3 and carbonized residues formed [23].
For 0 wt% ATP clay there is a sharp steep step with an onset temperature around 185 °C which increases by increasing ATP content recording 223 °C for 2 wt% ATP. The final residual yields are (18.5, 36.8, 35.9, 41.1 and 43 wt%) for (0, 0.25, 0.5, 1and 2 wt%), respectively. Also, it is remarked that, the total weight loss percentages after the third decomposition stage decreased from 81.5 to 57 wt% indicating that the beads with higher clay content have higher onset temperature, lower weight loss and hence higher residual yield than clay free beads. So, we can conclude that incorporation of small amount of ATP clay into PVA-alginate beads remarkably improve their thermal stability and prolonged their degradation time due to increasing the inorganic matter content or the clay content, which is thermally most stable and not decomposed. The same results were previously reported by Du et al. [69], who demonstrated that the thermal stability of poly acrylic acid composite membranes enhanced by increasing laponite concentration in the membranes. Kamoun et al. [17] also reported that the interaction between the polymers in the nanocomposite and clay as nano-filler might result in enhancing thermal decomposition.
GC–MS Analysis
The most suitable approach to measure bioremediation efficiency is to monitor the hydrocabons disappearance rates which can be demonstrated using GC–MS analysis as represented in Figs. 10 and 11. By comparing the crude oil control sample (Figs.10a and 11a) with the bioremediation metabolites ( Figs.10b and 11b) for Pseudomonas stutzeri and Rhodococcus qingshengii, respectively; it can be seen that after 28 days of incubation under the optimium conditions, the peaks associated with lighter hydrocarbons components with low retention times have been disapeared. Also Pseudomonas stutzeri and Rhodococcus qingshengii, the heavy compontents that represenet the long-chain hydrocarbons were broken down into lower intermidates and less complexity with lower concentrations that showed new appearing peaks. The same results were reported by Lee et al. [70] who reported that the long chain hydorcarbons have been disappeared after their biodegradation, and new peaks of short-chain hydrocarbons were appeared. Table 2 show examples of the major hydrocarbons identified in the crude oil sample that has been completely degraded after the bioremediation process. However, some of the high molecular weight hydrocarbons ranging from C15 to C44 were still present, but n-alkanes, iso-alkanes, alkenes and some aromatic hydrocarbons were sussessfully disappered in contrast to control sample. These results confirmed that, both Pseudomonas stutzeri and Rhodococcus qingshengii utilized different types of hydrocarbons as sole carbon and energy sources but Pseudomonas stutzeri showed slightly higher degradation potentail than Rhodococcus qingshengii bacterial isolate. Our results are consistent with Popoola et al. [71] and also Ilyas et al. [72] who demostrated the same results in their study of crude oil bioremediation.
All these results conclude the effectivness of the immobilization of the bacterial cells inside the PVA/Alginate/Attapulgite clay mineral clay that has been fully characterized and showed higher crude oil biodegradation capability compared with free bacterial cells.
Conclusions
In summary, crude oil degrading bacteria was successfully immobilized into PVA-alginate beads cross-linked with CaCl2 and physically reinforced by different types of clays especially ATP mineral clay. Also, the optimum conditions required for the isolated bacterial isolates for more efficient bioremediation process were studied and the obtained results proved that moderate shaking at 150 rpm and incubation at 30 °C at neutral pH are the most favorable conditions for our tested bacterial isolates in terms of the crude oil biodegradation. The prepared beads have been characterized using FTIR, TGA, and SEM. The GC–MS analyses of the tested crude oil before and after bacterial biodegradation proved the ability of the tested bacteria to degrade huge numbers of the existed hydrocarbons as sole carbon and energy sources. The results also proved that the incorporation of ATP clay into the polymer matrix increased their mechanical, thermal stability, and decreased their swelling ability in addition to improving the bioremediation efficiency. So, the above-mentioned optimum conditions are recommended as an ecofriendly approach to get rid of crude oil pollution in case of sudden leakage. We recommend more attentions to the use of different types of clays especially Hydroxyapatite and Laponite RD in the bioremediation process for future studies and improving the bioremediation efficiency of the bacterial isolates using different conditions.
Data Availability
No data was used for the research described in the article.
Abbreviations
- PVA:
-
Polyvinyl alcohol
- SA:
-
Sodium alginate
- PAHs:
-
Poly aromatic hydrocarbons
- SWR (%):
-
Swelling ratio percentage
- LB:
-
Luria Bertani broth
- BH:
-
Bushnell-Hass broth
- BEN:
-
Bentonite clay
- MMT:
-
Montmorillonite clay
- ATP:
-
Attapulgite clay
- LRD:
-
Laponite RD clay
- HAP:
-
Hydroxyapatite
References
Ejaz M, Zhao B, Wang X, Bashir S, Haider FU, Aslam Z, Khan MI, Shabaan M, Naveed M, Mustafa A (2021) Isolation and characterization of oil-degrading Enterobacter sp. from naturally hydrocarbon-contaminated soils and their potential use against the bioremediation of crude oil. Appl Sci 11:3504
Hassanshahian M, Emtiazi G, Kermanshahi R, Cappello S (2010) Comparison of oil degrading microbial communities in sediments from the Persian Gulf and Caspian. Sea Soil Sediment Contam 19:277–291
Abdeen Z, El-Sheshtawy HK, Moustafa Y (2014) Enhancement of crude oil biodegradation by immobilizing of different bacterial strains on porous PVA hydrogels or combining of them with their produced biosurfactants. J Pet Environ Biotechnol 5:1
Mehrotra T, Zaman MN, Prasad BB, Shukla A, Aggarwal S, Singh R (2020) Rapid immobilization of viable Bacillus pseudomycoides in polyvinyl alcohol/glutaraldehyde hydrogel for biological treatment of municipal wastewater. Environ Sci Pollut Res 27:9167–9180
Chen B, Yuan M, Qian L (2012) Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J Soils Sediments 12:1350–1359
Das M, Adholeya A (2015) Potential uses of immobilized bacteria, fungi, algae, and their aggregates for treatment of organic and inorganic pollutants in wastewater: water challenges and solutions on a global scale. ACS Publications, Washington, pp 319–337
Mehrotra T, Dev S, Banerjee A, Chatterjee A, Singh R, Aggarwal S (2021) Use of immobilized bacteria for environmental bioremediation: a review. J Environ Chem Eng 9:105920
Liao H, Liu Y, Wang Q, Duan W (2018) Structure and properties of porous poly (vinyl alcohol) hydrogel beads prepared through a physical–chemical crosslinking method. J Appl Polym Sci 135:46402
Zain NAM, Suhaimi MS, Idris A (2011) Development and modification of PVA–alginate as a suitable immobilization matrix. Process Biochem 46:2122–2129
Hu X, Long L, Gong T, Zhang J, Yan J, Xue Y (2020) Enhanced alginate-based microsphere with the pore-forming agent for efficient removal of Cu (II). Chemosphere 240:124860
Zommere Ž, Nikolajeva V (2017) Immobilization of bacterial association in alginate beads for bioremediation of oil-contaminated lands. Environ Exp Bot 15:105–111
Partovinia A, Rasekh B (2018) Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments. Crit Rev Environ Sci Technol 48:1–38
Sun Y, Lei C, Khan E, Chen S, Sang T, Lin D, Feng Y (2018) Aging effects on chemical transformation and metal(loid) removal by entrapped nanoscale zero-valent iron for hydraulic fracturing wastewater treatment. Sci Total Environ 615:498–507
Zuo L (2020) Bioremediation of crude-oil polluted soil using immobilized microbes. IOP Conf Ser 510(4):042047
Sakdapetsiri C, Kaokhum N, Pinyakong O (2021) Biodegradation of crude oil by immobilized Exiguobacterium sp. AO-11 and shelf life evaluation. Sci Rep 11(1):1–13
Cheng Y, Lin H, Chen Z, Megharaj M, Naidu R (2012) Biodegradation of crystal violet using Burkholderia vietnamiensis C09V immobilized on PVA–sodium alginate–kaolin gel beads. Ecotoxicol Environ Saf 83:108–114
Kamoun EA, Menzel H (2012) HES-HEMA nanocomposite polymer hydrogels: swelling behavior and characterization. J Polym Res 19:1–14
Zhu Z, Dai J, Liu Y, Sun H, Liang W, Li A (2015) Hydrophobic spongy attapulgite for absorption of organics and oils from water.
Mulamattathil SG, Bezuidenhout C, Mbewe M, Ateba CN (2014) Isolation of environmental bacteria from surface and drinking water in Mafikeng, South Africa, and characterization using their antibiotic resistance profiles. J Pathog. https://doi.org/10.1155/2014/371208
Mishra A, Saxena A, Singh SP (2019) Isolation and characterization of microbial strains from refinery effluent to screen their bioremediation potential. J Pure Appl Microbiol 13:2325–2332
Aljar MAA, Rashdan S, Abd El-Fattah A (2021) Environmentally friendly polyvinyl alcohol−alginate/bentonite semi-interpenetrating polymer network nanocomposite hydrogel beads as an efficient adsorbent for the removal of methylene blue from aqueous solution. Polymers 13:4000
Mollaei M, Abdollahpour S, Atashgahi S, Abbasi H, Masoomi F, Rad I, Lotfi AS, Zahiri HS, Vali H, Noghabi KA (2010) Enhanced phenol degradation by Pseudomonas sp. SA01: gaining insight into the novel single and hybrid immobilizations. J Hazard mater 175(1–3):284–292
Baigorria E, Cano LA, Sanchez LM, Alvarez VA, Ollier RP (2020) Bentonite-composite polyvinyl alcohol/alginate hydrogel beads: preparation, characterization and their use as arsenic removaldevices. Environ Nanotechnol Monit Manag 14:100364
Narra K, Dhanalekshmi U, Rangara G, Raja D, Kuma CS, Reddy PN, Mandal AB (2012) Effect of formulation variables on rifampicin loaded alginate beads. Iran J Pharm Res 11(3):715
Cunningham C, Ivshina I, Lozinsky V, Kuyukina M, Philp J (2004) Bioremediation of diesel-contaminated soil by microorganisms immobilised in polyvinyl alcohol. Int Biodeterior Biodegradation 54:167–174
Wang Y, Liu M, Ni B, Xie L (2012) κ-Carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind Eng Chem Res 51:1413–1422
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11(1):1–42
Simarro R, González N, Bautista LF, Sanz R, Molina MC (2011) Optimisation of key abiotic factors of PAH (naphthalene, phenanthrene and anthracene) biodegradation process by a bacterial consortium. Water Air Soil Pollut 217:365–374
Das A, Dey A (2020) P-nitrophenol-bioremediation using potent Pseudomonas strain from the textile dye industry effluent. J Environ Chem Eng 8:103830
Wong J, Lai K, Wan C, Ma K, Fang M (2002) Isolation and optimization of PAH-degradative bacteria from contaminated soil for PAHs bioremediation. Water Air Soil Pollut 139:1–13
Al-Hadithi H, Al-Razzaq E, Fadhil G (2017) Bioremediation of polycyclic aromatic hydrocarbons by Acinetobacter species isolated from ecological sources. J Environ Biol 38:785
Fatajeva E, Gailiūtė I, Paliulis D, Grigiškis S (2014) The use of Acinetobacter sp. for oil hydrocarbon degradation in saline waters. Biologija. https://doi.org/10.6001/biologija.v60i3.2971
Huang S, Wang Y, Tang C, Jia H, Wu L (2021) Speeding up selenite bioremediation using the highly selenite-tolerant strain Providencia rettgeri HF16-A novel mechanism of selenite reduction based on proteomic analysis. J Hazard Mater 406:124690
Tekere M, Jacob-Lopes E, Zepka LQ (2019) Microbial bioremediation and different bioreactors designs applied. Biotechnol Bioeng. https://doi.org/10.5772/intechopen.83661
Abdel-Razek A, El-Sheikh H, Suleiman W, Taha TH, Mohamed M (2020) Bioelimination of phenanthrene using degrading bacteria isolated from petroleum soil: safe approach. Desalin Water Treat 181:131–140
Abtahi H, Parhamfar M, Saeedi R, Villasenor J, Sartaj M, Kumar V, Coulon F, Parhamfar M, Didehdar M, Koolivand A (2020) Effect of competition between petroleum-degrading bacteria and indigenous compost microorganisms on the efficiency of petroleum sludge bioremediation: field application of mineral-based culture in the composting process. J Environ Manage 258:110013
El-Liethy MA, El-Noubi MM, A.L.K Abia,et al. (2022) Eco-friendly bioremediation approach for crude oil-polluted soils using a novel and biostimulated Enterobacter hormaechei ODB H32 strain. Int J Environ Sci Technol 19(11):10577–10588
Hazim RN, Al-Ani MA (2019) Effect of petroleum hydrocarbons contamination on soil microorganisms and biodegradation. Rafidain J Sci 28:13–22
Abarian M, Hassanshahian M, Esbah A (2019) Degradation of phenol at high concentrations using immobilization of Pseudomonas putida P53 into sawdust entrapped in sodium-alginate beads. Water Sci Technol 79(7):1387–1396
Ibrahim IM, Konnova SA, Sigida EN, Lyubun EV, Muratova AY, Fedonenko YP, Elbanna К (2020) Bioremediation potential of a halophilic Halobacillus sp. strain, EG1HP4QL: exopolysaccharide production, crude oil degradation, and heavy metal tolerance. Extremophiles 24:157–166
Sed A (2015) Biodegradation of Phenanthrene by Klebsiella sp. isolated from organic contaminated sediment. J Adv Biol Biotechnol 4:1–12
Baoune H, Aparicio JD, Pucci G, Ould El Hadj-Khelil A, Polti MA (2019) Bioremediation of petroleum-contaminated soils using Streptomyces sp. Hlh1. J Soils Sediments 19:2222–2230
El-Sheshtawy H, Aman D, Nassar H (2022) A novel bioremediation technique for petroleum hydrocarbons by bacterial consortium immobilized on goethite-chitosan nanocomposite. Soil Sediment Contam 31:176–199
Usmani Z, Kulp M, Lukk T (2021) Bioremediation of lindane contaminated soil: exploring the potential of actinobacterial strains. Chemosphere 278:130468
Xu L, Zhu L (2009) Structures of OTMA-and DODMA-bentonite and their sorption characteristics towards organic compounds. J Colloid Interface Sci 331:8–14
Acikyildiz M, Gurses A, Yolcu H (2015) Synthesis of super hydrophobic clay by solution intercalation method from aqueous dispersions. Acta Phys Pol A 127:1156–1160
Warr LN, Perdrial JN, Lett MC, Heinrich-Salmeron A, Khodja M (2009) Clay mineral-enhanced bioremediation of marine oil pollution. Appl Clay Sci 46(4):337–345
Churchman GJ, Gates WP, Theng BKG, Yuan G (2006) Clays and clay minerals for pollution control. Develop Clay Sci 1:625–675
Murray HH (2000) Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Appl Clay Sci 17(5–6):207–221
Owens EH, Lee K (2003) Interaction of oil and mineral fines on shorelines: review and assessment. Mar Pollut Bull 47(9–12):397–405
Elbassyoni S, Kamoun EA, Taha TH, Rashed MA, ElNozahi FA (2020) Effect of Egyptian attapulgite clay on the properties of PVA-HES–clay nanocomposite hydrogel membranes for wound dressing applications. Arab J Sci Eng 45:4737–4749
Ruan B, Wu P, Chen M, Lai X, Chen L, Yu L, Gong B, Kang C, Dang Z, Shi Z (2018) Immobilization of Sphingomonas sp. GY2B in polyvinyl alcohol–alginate–kaolin beads for efficient degradation of phenol against unfavorable environmental factors. Ecotoxicol Environ Saf 162:103–111
Lin H, Chen Z, Megharaj M, Naidu R (2013) Biodegradation of TNT using Bacillus mycoides immobilized in PVA–sodium alginate–kaolin. Appl Clay Sci 83:336–342
Costa-Gutierrez SB, Aparicio JD, Delgado OD, Benimeli CS, Polti MA (2021) Use of glycerol for the production of actinobacteria with well-known bioremediation abilities. 3 Biotech 11:1–10
Philip L (2006) Bioremediation of endosulfan contaminated soil and water—optimization of operating conditions in laboratory scale reactors. J Hazard Mater 136:354–364
Gouda MK, Omar SH, Chekroud ZA, Eldin HMN (2007) Bioremediation of kerosene I: a case study in liquid media. Chemosphere 69:1807–1814
Talha MA, Goswami M, Giri B, Sharma A, Rai B, Singh R (2018) Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Biores Technol 252:37–43
Padmanaban V, Geed SR, Achary A, Singh R (2016) Kinetic studies on degradation of reactive red 120 dye in immobilized packed bed reactor by Bacillus cohnii RAPT1. Biores Technol 213:39–43
Bayat Z, Hassanshahian M, Cappello S (2015) Immobilization of microbes for bioremediation of crude oil polluted environments: a mini review. Open Microbiol J 9:48–54
Knijnenburg JT, Kasemsiri P, Amornrantanaworn K, Suwanree S, Iamamornphan W, Chindaprasirt P, Jetsrisuparb K (2021) Entrapment of nano-ZnO into alginate/polyvinyl alcohol beads with different crosslinking ions for fertilizer applications. Int J Biol Macromol 181:349–356
Omidian H, Rocca JG, Park K (2006) Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate. Macromol Biosci 6:703–710
Nunes MA, Vila-Real H, Fernandes PC, Ribeiro MH (2010) Immobilization of naringinase in PVA–alginate matrix using an innovative technique. Appl Biochem Biotechnol 160:2129–2147
Golafshan N, Rezahasani R, Esfahani MT, Kharaziha M, Khorasani S (2017) Nanohybrid hydrogels of laponite: PVA-Alginate as a potential wound healing material. Carbohyd Polym 176:392–401
Mahdavinia GR, Mousanezhad S, Hosseinzadeh H, Darvishi F, Sabzi M (2016) Magnetic hydrogel beads based on PVA/sodium alginate/laponite RD and studying their BSA adsorption. Carbohyd Polym 147:379–391
Shivakumara LR, Demappa T (2019) Synthesis and swelling behavior of sodium alginate/poly (vinyl alcohol) hydrogels. Turk J Pharm Sci 16:252
Hussein Y, El-Fakharany EM, Kamoun EA, Loutfy SA, Amin R, Taha TH, Salim SA, Amer M (2020) Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: nanofibers optimization and in vitro bioevaluation. Int J Biol Macromol 164:667–676
Rahman N, Wilfred CD (2018) Removal of Mn (VII) from industrial wastewater by using alginate-poly (vinyl) alcohol as absorbent. J Phys. https://doi.org/10.1088/1742-6596/1123/1/012067
Levic S, Djordjevic V, Rajic N, Milivojevic M, Bugarski B, Nedovic V (2013) Entrapment of ethyl vanillin in calcium alginate and calcium alginate/poly (vinyl alcohol) beads. Chem Pap 67:221–228
Du J, Zhu J, Wu R, Xu S, Tan Y, Wang J (2015) A facile approach to prepare strong poly (acrylic acid)/LAPONITE® ionic nanocomposite hydrogels at high clay concentrations. RSC Adv 5:60152–60160
Lee DW, Lee H, Kwon B-O, Khim JS, Yim UH, Kim BS, Kim J-J (2018) Biosurfactant-assisted bioremediation of crude oil by indigenous bacteria isolated from Taean beach sediment. Environ Pollut 241:254–264
Popoola LT, Yusuff AS (2021) Optimization and characterization of crude oil contaminated soil bioremediation using bacteria isolates: plant growth effect, South African. J Chem Eng 37:206–213
Saeed M, Ilyas N, Arshad M, Sheeraz M, Ahmed I, Bhattacharya A (2021) Development of a plant microbiome bioremediation system for crude oil contamination. J Environ Chem Eng 9:105401
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no specific fund was received for conducting this research.
Author information
Authors and Affiliations
Contributions
EF conducted the experimental part and written the original and final draft; EAK and AED: study design, characterization and supervision and reviewed the original manuscript; THT: experimental design, microbiology experiments and supervision; and TK: Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farid, E., Kamoun, E.A., Taha, T.H. et al. Eco-friendly Biodegradation of Hydrocarbons Compounds from Crude Oily Wastewater Using PVA/Alginate/Clay Composite Hydrogels. J Polym Environ 32, 225–245 (2024). https://doi.org/10.1007/s10924-023-02991-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02991-y