Abstract
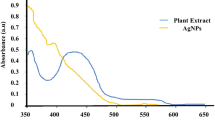
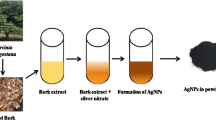
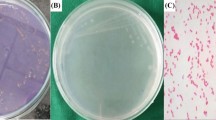
Advent of nanotechnology opens up potential avenues for overcoming various challenges that includes control and cure of infectious diseases. The present study is focused on the synthesis of α-chitin nanoparticles (CNP) from the shells of Penaeus monodon Fabricius, silver nanoparticles (AgNP) and α-chitin/silver nanocomposite (CNP/AgNP), and to evaluate their antimicrobial and mosquito larvicidal activities. The antibacterial and antifungal activities are assessed against five different bacterial and fungal strains. Also the mosquito larvicidal potential is studied against Aedes aegypti a potential vector for malaria and dengue fever. The synthesized nanocomposites are characterized using UV–Vis spectroscopy, X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscope, energy-dispersive X-ray spectroscopy, transmission electron microscope and dynamic light scattering analysis. The antibacterial and antifungal assays reveal that the CNP/AgNP has an enhanced antimicrobial effect on inhibition of bacteria (P. vulgaris, K. pneumonia and S. aureus) as well as fungi (C. albicans, T. viridae, A. niger and A. alternate). Mosquito larvicidal assays confirm that CNP/AgNP has shown lowest LC50 and LC90 values than CNP and AgNP against all the instars of A. aegypti. Hence our result suggests that the incorporation of AgNP with CNP could improve the antimicrobial and mosquito larvicidal activity and have the potential to be used as biocompatible antimicrobial and mosquito larvicidal material.









Similar content being viewed by others
References
Wang S, Bai J, Li C, Zhang Y, Zhang J (2012) Ag nanoparticle-embedded one-dimensional β-CD/PVP composite nanofibers prepared via electrospinning for use in antibacterial material. Colloid Polym Sci 290(7):667–672
Nagarajan S, Mohana M, Sudhagar P, Raman V, Nishimura T, Kim S, Rajendran N (2012) Nanocomposite coatings on biomedical grade stainless steel for improved corrosion resistance and biocompatibility. ACS Appl Mater Interfaces 4(10):5134–5141
Pandian CJ, Palanivel R, Dhananasekaran S (2015) Green synthesis of nickel nanoparticles using Ocimum sanctum and their application in dye and pollutant adsorption. Chin J Chem Eng 23(8):1307–1315
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4(3):707–716
Shukla S, Kim KT, Baev A, Yoon YK, Litchinitser NM, Prasad PN (2010) Fabrication and characterization of gold–polymer nanocomposite plasmonic nanoarrays in a porous alumina template. ACS Nano 4(4):2249–2255
Shenhar R, Norsten TB, Rotello VM (2005) Polymer-mediated nanoparticle assembly: structural control and applications. Adv Mater 17(6):657–669
Jayakumar R, Prabaharan M, Kumar PS, Nair SV, Tamura H (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29(3):322–337
Shahidi F, Abuzaytoun R (2005) Chitin, chitosan, and co-products: chemistry, production, applications, and health effects. Adv Food Nut Res 49:93–137
Aranaz I, Mengíbar M, Harris R, Paños I, Miralles B, Acosta N, Heras Á (2009) Functional characterization of chitin and chitosan. Curr Chem Biol 3(2):203–230
Jayakumar R, Chennazhi K, Muzzarelli R, Tamura H, Nair S, Selvamurugan N (2010) Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr Polym 79(1):1–8
Tamura H, Furuike T, Nair SV, Jayakumar R (2011) Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr Polym 84(2):820–824
Pandian CJ, Palanivel R, Dhanasekaran S (2016) Screening antimicrobial activity of nickel nanoparticles synthesized using Ocimum sanctum leaf extract. J Nanopart 2016:1–13
Khalil MM, Ismail EH, El-Baghdady KZ, Mohamed D (2014) Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J Chem 7(6):1131–1139
WHO fact sheet (updated March 2014) for vector borne diseases. http://www.who.int/campaigns/world-health-day/2014/fact-sheets/en/336
Raghavendra K, Subbarao S (2002) Chemical insecticides in malaria vector control in India. ICMR Bull 32(10):1–7
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008) Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol Res 102(5):981–988
Solairaj D, Palanivel R, Pappu S (2015) Adsorption of methylene blue, bromophenol blue and coomassie brilliant blue by α-chitin nanoparticles. J Adv Res 7(1):113–124
Deng W, Jin D, Drozdowicz-Tomsia K, Yuan J, Wu J, Goldys EM (2011) Ultrabright Eu-doped plasmonic Ag@ SiO2 nanostructures: time-gated bioprobes with single particle sensitivity and negligible background. Adv Mater 23(40):4649–4654
Singh R, Singh AK, Soam A, Shahi SK (2013) Antifungal screening of various spice extracts on azole resistant strains of Candida. Curr Discov 2(1):46–51
Lokina S, Stephen A, Kaviyarasan V, Arulvasu C, Narayanan V (2014) Cytotoxicity and antimicrobial activities of green synthesized silver nanoparticles. Eur J Med Chem 76:256–263
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Miller G (1959) Use of dinitrisalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–429
Chen CZ, Cooper SL (2002) Interactions between dendrimer biocides and bacterial membranes. Biomaterials 23(16):3359–3368
Li WR, Xie XB, Shi QS, Zeng HY, You-Sheng OY, Chen YB (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biot 85(4):1115–1122
Broekaert WF, Terras FR, Cammue BP, Vanderleyden J (1990) An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett 69(1–2):55–59
de Oliveira Pereira F, Mendes JM, de Oliveira Lima E (2013) Investigation on mechanism of antifungal activity of eugenol against Trichophyton rubrum. Med Mycol 51(5):507–513
Manilal A, Thajuddin N, Selvin J, Idhayadhulla A, Kumar RS, Sujith S (2011) In vitro mosquito larvicidal activity of marine algae against the human vectors, Culex quinquefasciatus (Say) and Aedes aegypti (Linnaeus) (Diptera: Culicidae). Int J Zool Res 7(3):272–278
Koodalingam A, Mullainadhan P, Arumugam M (2011) Effects of extract of soapnut Sapindus emarginatus on esterases and phosphatases of the vector mosquito, Aedes aegypti (Diptera: Culicidae). Acta Trop 118(1):27–36
Nathan SS, Choi MY, Paik CH, Seo HY (2007) Food consumption, utilization, and detoxification enzyme activity of the rice leaffolder larvae after treatment with Dysoxylum triterpenes. Pestic Biochem Phys 88(3):260–267
Pandian CJ, Palanivel R, Dhanasekaran S (2015) Green synthesis of nickel nanoparticles using Ocimum sanctum and their application in dye and pollutant adsorption. Chin J Chem Eng 23(8):1307–1315
El-Kheshen AA, El-Rab SFG (2012) Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Der Pharma Chem 4(1):53–65
Madhumathi K, Kumar PS, Abhilash S, Sreeja V, Tamura H, Manzoor K, Jayakumar R (2010) Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J Mater Sci Mater Med 21(2):807–813
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339(16):2693–2700
Kumar PS, Abhilash S, Manzoor K, Nair SV, Tamura H, Jayakumar R (2010) Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr Polym 80(3):761–767
Nguyen VQ, Ishihara M, Mori Y, Nakamura S, Kishimoto S, Hattori H, Matsui T (2013) Preparation of size-controlled silver nanoparticles and chitin-based composites and their antimicrobial activities. J Nanomater. doi:10.1155/2013/693486
Langfield RD, Scarano FJ, Heitzman ME, Kondo M, Hammond GB, Neto CC (2004) Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. J Ethnopharmacol 94(2):279–281
Agnihotri S, Mukherji S, Mukherji S (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv 4(8):3974–3983
Losasso C, Belluco S, Cibin V, Zavagnin P, Mičetić I, Gallocchio F, Ricci A (2014) Antibacterial activity of silver nanoparticles: sensitivity of different Salmonella serovars. Front Microbiol 5:227
Li WR, Xie XB, Shi QS, Duan SS, Ouyang YS, Chen YB (2011) Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals 24(1):135–141
Li WR, Xie XB, Shi QS, Zeng HY, You-Sheng OY, Chen YB (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85(4):1115–1122
Cai X, Zhang B, Liang Y, Zhang J, Yan Y, Chen X, Wu T (2015) Study on the antibacterial mechanism of copper ion-and neodymium ion-modified α-zirconium phosphate with better antibacterial activity and lower cytotoxicity. Colloid Surface B 132:281–289
Wu T, Xie AG, Tan SZ, Cai X (2011) Antimicrobial effects of quaternary phosphonium salt intercalated clay minerals on Escherichia coli and Staphylococci aureus. Colloid Surface B 86(1):232–236
Grigor’eva A, Saranina I, Tikunova N, Safonov A, Timoshenko N, Rebrov A, Ryabchikova E (2013) Fine mechanisms of the interaction of silver nanoparticles with the cells of Salmonella typhimurium and Staphylococcus aureus. Biometals 26(3):479–488
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18(22):1–9
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275(1):177–182
Salaberria AM, Fernandes SC, Diaz RH, Labidi J (2015) Processing of α-chitin nanofibers by dynamic high pressure homogenization: characterization and antifungal activity against A. niger. Carbohydr Polym 116:286–291
Krishnaraj C, Ramachandran R, Mohan K, Kalaichelvan PT (2012) Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim Acta A 93:95–99
Ouda SM (2014) Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res J Microbiol 9(1):34
Mei L, Lu Z, Zhang X, Li C, Jia Y (2014) Polymer-Ag nanocomposites with enhanced antimicrobial activity against bacterial infection. ACS Appl Mater Interfaces 6(18):15813–15821
Roopan SM, Madhumitha G, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV (2013) Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crop Prod 43:631–635
Lemos FJ, Cornel AJ, Jacobs-Lorena M (1996) Trypsin and aminopeptidase gene expression is affected by age and food composition in Anopheles gambiae. Insect Biochem Mol Biol 26(7):651–658
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45(1):371–391
Suryawanshi RK, Patil CD, Borase HP, Narkhede CP, Salunke BK, Patil SV (2015) Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic Biochem Phys 123:49–55
Koodalingam A, Mullainadhan P, Rajalakshmi A, Deepalakshmi R, Ammu M (2012) Effect of a Bt-based product (Vectobar) on esterases and phosphatases from larvae of the mosquito Aedes aegypti. Pestic Biochem Phys 104(3):267–272
Acknowledgments
We are grateful to the University grants commission, New Delhi, India for the financial assistance of this study through a major research project (F. No. 40-389/2011(SR)). Thanks are also due to the Center for Research in Medical Entomology, Madurai, Tamil Nadu, India for providing the eggs of A. aegypti.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Solairaj, D., Rameshthangam, P. Silver Nanoparticle Embedded α-Chitin Nanocomposite for Enhanced Antimicrobial and Mosquito Larvicidal Activity. J Polym Environ 25, 435–452 (2017). https://doi.org/10.1007/s10924-016-0822-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0822-3