Abstract
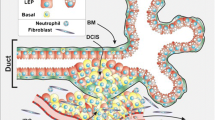
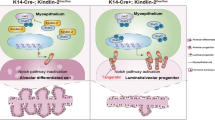
Until recently, myoepithelial cells—the second major cell population in the mammary epithelium—were not considered to play an important role in the morphogenetic events during gland development. Mouse mutants with changes in the gene expression pattern characteristic of the basal myoepithelial cell layer have been generated and used to show that these cells influence the proliferation, survival and differentiation of luminal cells, modulate stromal–epithelial interactions and actively participate in mammary morphogenesis. Various cellular and molecular mechanisms may underlie the observed phenotypes. These include an unbalanced expression of matrix degrading metalloproteinases (MMPs) and their inhibitors, leading to changes in the composition and organization of the (extracellular matrix) ECM, the production of soluble growth factors affecting stromal and epithelial cell growth and differentiation and direct signaling through cell–cell contacts between the myoepithelial and luminal cell layers.
Similar content being viewed by others
Abbreviations
- CEBP-β:
-
CCAAT enhancer-binding protein β
- COX-2:
-
cyclooxygenase-2
- ECM:
-
extracellular matrix
- EphB4:
-
ephrin receptor B4
- K5:
-
K8, K14, K18, cytokeratin 5, 8, 14 and 18, respectively
- LAP:
-
liver-enriched transcriptional activating protein
- Lef:
-
lymphocyte enhancer factor
- LIP:
-
liver-enriched transcriptional inhibitory protein
- MMP:
-
matrix metalloproteinase
- MMTV:
-
mouse mammary tumor virus
- PTHrP:
-
parathyroid hormone-related protein
- Tcf:
-
T-cell factor
- TEB:
-
terminal end bud
- TIMP:
-
tissue inhibitor of metalloproteinases
- WAP:
-
whey acidic protein
References
Deugnier MA, Teuliere J, Faraldo MM, Thiery JP, Glukhova MA. The importance of being a myoepithelial cell. Breast Cancer Res 2002;4:224–30.
Gusterson BA, Ross DT, Heath VJ, Stein T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res 2005;7:143–8.
Adriance MC, Inman JL, Petersen OW, Bissell MJ. Myoepithelial cells: Good fences make good neighbors. Breast Cancer Res 2005;7:190–7.
Sternlicht MD, Barsky SH. The myoepithelial defense: A host defense against cancer. Med Hypotheses 1997;48:37–46.
Lakhani SR, O’Hare MJ. The mammary myoepithelial cell–Cinderella or ugly sister? Breast Cancer Res 2001;3:1–4.
Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 2003;71:1–17.
Deugnier MA, Moiseyeva EP, Thiery JP, Glukhova M. Myoepithelial cell differentiation in the developing mammary gland: Progressive acquisition of smooth muscle phenotype. Dev Dyn 1995;204:107–17.
Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 2005;6:622–34.
Daniel CW, Strickland P, Friedmann Y. Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev Biol 1995;169:511–9.
Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol 1997;139:1025–32.
Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol 2003;15:763–70.
Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev 2003;17:1189–200.
Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol 2004;48:477–87.
Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Clevers H. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 1999;285:1923–6.
Barker N, Huls G, Korinek V, Clevers H. Restricted high level expression of Tcf-4 protein in intestinal and mammary gland epithelium. Am J Pathol 1999;154:29–35.
Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci 2005;118:3585–94.
Hinck L. The versatile roles of “axon guidance” cues in tissue morphogenesis. Dev Cell 2004;7:783–93.
Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell 2003;4:371–82.
Koster MI, Roop DR. p63 and epithelial appendage development. Differentiation 2004;72:364–70.
Westfall MD, Pietenpol JA. p63: Molecular complexity in development and cancer. Carcinogenesis 2004;25:857–64.
McKeon F. p63 and the epithelial stem cell: More than status quo? Genes Dev 2004;18:465–9.
Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999;398:708–13.
Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999;398:714–8.
Keyes WM, Wu Y, Vogel H, Guo X, Lowe SW, Mills AA. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev 2005;19:1986–99.
Senoo M, Matsumura Y, Habu S. TAp63gamma (p51A) and dNp63alpha (p73L), two major isoforms of the p63 gene, exert opposite effects on the vascular endothelial growth factor (VEGF) gene expression. Oncogene 2002;21:2455–65.
Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, Lee CC, Dong SM, Guo Z, Benoit N, Cohen Y, Rechthand P, Califano J, Moon CS, Ratovitski E, Jen J, Sidransky D, Trink B. DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res 2003;63:2351–7.
Taddei I, Faraldo MM, Teuliere J, Deugnier MA, Thiery JP, Glukhova MA. Integrins in mammary gland development and differentiation of mammary epithelium. J Mammary Gland Biol Neoplasia 2003;8:383–94.
Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol 1996;175:301–13.
Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 2001;29:418–25.
Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol 2001;153:555–68.
Michaelson JS, Leder P. beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland. Oncogene 2001;20:5093–9.
Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E, Xu X, Seldin DC, Schmidt EV, Taketo MM, Robinson GW, Cardiff RD, Hennighausen L. Activation of different Wnt/beta-catenin signaling components in mammary epithelium induces transdifferentiation and the formation of pilar tumors. Oncogene 2002;21:5548–56.
Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze’ev A, Thiery JP, Glukhova MA. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development 2005;132:267–77.
Escande B, Lindner V, Massfelder T, Helwig JJ, Simeoni U. Developmental aspects of parathyroid hormone-related protein biology. Semin Perinatol 2001;25:76–84.
Wysolmerski JJ, McCaughern-Carucci JF, Daifotis AG, Broadus AE, Philbrick WM. Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development 1995;121:3539–47.
Dunbar ME, Dann P, Brown CW, Van Houton J, Dreyer B, Philbrick WP, Wysolmerski JJ. Temporally regulated overexpression of parathyroid hormone-related protein in the mammary gland reveals distinct fetal and pubertal phenotypes. J Endocrinol 2001;171:403–16.
Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM. Cyclooxygenases in cancer: progress and perspective. Cancer Lett 2004;215:1–20.
Wang D, Dubois RN. Cyclooxygenase-2: A potential target in breast cancer. Semin Oncol 2004;31:64–73.
Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 2001;276:18563–9.
Muller-Decker K, Berger I, Ackermann K, Ehemann V, Zoubova S, Aulmann S, Pyerin W, Furstenberger G. Cystic duct dilatations and proliferative epithelial lesions in mouse mammary glands upon keratin 5 promoter-driven overexpression of cyclooxygenase-2. Am J Pathol 2005;166:575–84.
Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci 1998;111:2741–51.
Munarini N, Jager R, Abderhalden S, Zuercher G, Rohrbach V, Loercher S, Pfanner-Meyer B, Andres AC, Ziemiecki A. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci 2002;115:25–37.
Hirai Y, Radisky D, Boudreau R, Simian M, Stevens ME, Oka Y, Takebe K, Niwa S, Bissell MJ. Epimorphin mediates mammary luminal morphogenesis through control of C/EBPbeta. J Cell Biol 2001;153:785–94.
Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 1994;263:526–9.
Streuli CH. Maspin is a tumour suppressor that inhibits breast cancer tumour metastasis in vivo. Breast Cancer Res 2002;4:137–40.
Zhang M, Magit D, Botteri F, Shi HY, He K, Li M, Furth P, Sager R. Maspin plays an important role in mammary gland development. Dev Biol 1999;215: 278–87.
Radice GL, Sauer CL, Kostetskii I, Peralta Soler A, Knudsen KA. Inappropriate P-cadherin expression in the mouse mammary epithelium is compatible with normal mammary gland function. Differentiation 2003;71:361–73.
Cardiff RD. The biology of mammary transgenes: five rules. J Mammary Gland Biol Neoplasia 1996;1:61–73.
Siegel PM, Hardy WR, Muller WJ. Mammary gland neoplasia: Insights from transgenic mouse models. Bioessays 2000;22:554–63.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100:3983–8.
Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer 2003;3:832–44.
Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 2004;14:43–7.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–74.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–74.
Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 1997;3:1949–58.
Nguyen M, Lee MC, Wang JL, Tomlinson JS, Shao ZM, Alpaugh ML, Barsky SH. The human myoepithelial cell displays a multifaceted anti-angiogenic phenotype. Oncogene 2000;19:3449–59.
Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004;6:17–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faraldo, M.M., Teulière, J., Deugnier, MA. et al. Myoepithelial Cells in the Control of Mammary Development and Tumorigenesis: Data From Genetically Modified Mice. J Mammary Gland Biol Neoplasia 10, 211–219 (2005). https://doi.org/10.1007/s10911-005-9582-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-005-9582-8