Abstract
The mammary gland consists of an extensively branched ductal network contained within a distinctive basement membrane and encompassed by a stromal compartment. During lactation, production of milk depends on the action of the two epithelial cell types that make up the ductal network: luminal cells, which secrete the milk components into the ductal lumen; and myoepithelial cells, which contract to aid in the ejection of milk. There is increasing evidence that the myoepithelial cells also play a key role in the organizational development of the mammary gland, and that the loss and/or change of myoepithelial cell function is a key step in the development of breast cancer. In this review we briefly address the characteristics of breast myoepithelial cells from human breast and mouse mammary gland, how they function in normal mammary gland development, and their recently appreciated role in tumor suppression.
Similar content being viewed by others
Introduction
The mammary ductal tree is a bilayered structure that consists of an iterative repetition of basic functional elements. However, when comparing the mouse and human mammary glands, differences emerge. In the mouse the mammary epithelial cells are encased by a periductal stroma that is surrounded by fat tissue, whereas human breast epithelial cells are directly encompassed by highly vascularized intralobular loose connective tissue, and are separated from the adipose tissue by dense interlobular fibrous connective tissue [1]. Moreover, in the mouse, branching ducts terminate in end buds that differentiate during pregnancy and lactation into lobular acini (for review [2]), whereas the human breast exhibits a higher level of differentiation, with terminal ductal lobular units present in the resting state; these lobular acini differentiate further during pregnancy and lactation to secrete milk (for review [1]).
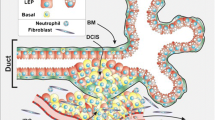
The ductal network in both mouse and human is comprised of two epithelial cell types: luminal epithelial and myoepithelial cells. Ductal myoepithelial cells are spindle shaped and oriented parallel to the long axis such that they form a continuous layer around the luminal cells, especially in the ducts (Fig. 1); upon contraction the myoepithelial cells decrease the length and increase the diameter of the ducts to eject the milk [3]. In contrast, acinar myoepithelial cells are stellate shaped, forming a discontinuous basket-like network around the luminal cells, although during pregnancy and lactation the myoepithelial cell body and processes extend to fully encompass the expanded alveolar epithelial cells [3]. Functionally, myoepithelial cells are a hybrid of both smooth muscle ('myo') and epithelial cells (Table 1). Like muscle cells, myoepithelial cells express filamentous smooth muscle actin and smooth muscle myosin, and exhibit contractile properties; like epithelial cells, myoepithelial cells express intermediate filaments (the epithelial keratins) [4–6] and have cadherin-mediated cell–cell junctions [1, 4, 7, 8]. Structurally, myoepithelial cells form distinct desmosomes with both luminal cells and other myoepithelial cells, generate gap junctions and cadherin–cadherin interactions with other myoepithelial cells, and adhere to the basement membrane (BM) via hemidesmosomes [9–12].
Cross-section of a bilayered duct. Secretory luminal cells (LEPs) are apically located to contractile myoepithelial cells (MEPs) and the basement membrane (BM). Milk proteins and cleaved Muc1 are secreted into the luminal space during lactation. Desmosomes containing desmoglein (Dsg)2 and desmocollin (Dsc)2 form between adjacent luminal cells and between adjacent LEPs and MEPs. Desmosomes between MEPs contain Dsg3 and Dsc3. MEPs as contractile cells contain smooth muscle actin and adhere to the BM via hemidesmosomes.
The structural and functional elements of myoepithelial cells are inextricably linked. During lactation, myoepithelial cells contract in response to oxytocin and move milk into the ducts (for review [13]), and gap junctions and cadherin-based interactions connecting myoepithelial cells function to coordinate the ejection of milk smoothly (for review [14]). During development, myoepithelial cells also act to induce luminal cell polarity [5, 15] and to regulate ductal morphogenesis [16]; here, connection to the BM and the desmosomal interactions with the luminal epithelial cells facilitate paracrine regulatory mechanisms. Proper coordination of all of these activities is necessary to maintain normal breast function; accordingly, it is unsurprising that the loss of myoepithelial function is almost universally associated with breast cancer [1, 15, 17].
Myoepithelial function in normal breast
The functional interactions that define the bilayered acinus have been explored using three-dimensional culture systems. When phenotypically normal human or rodent luminal cells are grown in laminin-rich extracellular matrix (lrECM) gels, they recreate the structure and function of the acinus found in vivo even in the absence of myoepithelial cells [6, 18]. We believe that this is possible, in part, because cultured luminal cells express a number of proteins that are characteristic of myoepithelial cells in vivo (e.g. β4 integrin [10], epidermal growth factor receptor [19], vimentin [20], maspin [21], and others; for review [1]). It may be that luminal cells can form acinar structures in culture because of this ability to become luminal/myoepithelial 'hybrids'. The possibility that expression of specific myoepithelial proteins confers distinctive signaling cues that promote cell survival and proper apicobasal polarity is an active area of investigation in our laboratory and those of our collaborators [15, 18, 22, 23].
Of the molecules produced by myoepithelial cells to regulate luminal cell function, laminin-1 and desmosomal proteins have emerged as key mediators. Laminin-1 is a heterotrimer of α1, β1 and γ1 chains, and is a major component of BM (for review [24]). Embryos derived from murine embryonic stem cells null for the laminin-1 β1 and γ1 chains are embryonically lethal at day 5.5 and lack BM [25, 26]. Interestingly, embryos derived from murine embryonic stem cells null for the laminin-1 α1 chain or the α1 LG4-5 domains are also embryonically lethal; however, these null embryos do form an embryonic BM, possibly because of compensation by the α5 chain from laminin-10 (α5β1γ1) [26–28].
Cell/laminin-1 interactions were previously implicated in tissue morphogenesis and maintenance of polarity in kidney, salivary gland, and intestine and mammary epithelial cells [29–32], and we showed that interactions with laminin-1 are important for the functional mammary cell differentiation to produce the milk protein β-casein [33]. Disruption of signaling by β1 integrin inhibitory antibodies or by the E-3 fragment of laminin-1 inhibits the expression of β-casein [33, 34], and subsequent experiments have suggested that organized polymerization of laminin-1 is required for functional mammary differentiation [35–37]. We previously showed that human breast luminal cells, when grown in three-dimensional type I collagen as opposed to laminin-rich gels, form structures with altered integrins [38] that have reversed polarity and lack central lumina [15]; however, if these same cells are cocultured with myoepithelial cells in collagen I gels they exhibit correct apicobasal polarity, as they do when cultured in lrECM gel [15] (for review [39]). It was revealed that the myoepithelial cells are the only epithelial cells in the breast that produce the α1 chain of laminin-1, and thus they are a key determinant for correct luminal cell polarization in three-dimensional collagen [15]. Although the experiments described above demonstrated that laminin-1 could direct the formation of acinar structures in three-dimensional cultures, it was not clear whether laminin-1 was the molecule that directed this morphogenic process in vivo or whether other molecules are also involved.
Parallel studies by others using a rotary culture system have suggested an alternative solution in which cell–cell adhesion may be the ultimate regulator for establishment of the acinar structure (Fig. 2). Runswick and coworkers found that inhibition of myoepithelial-specific desmosomal cadherins, desmocollin 3 (Dsc 3) desmoglein 3 (Dsg 3), prevented morphogenesis of the bilayered acinus structure and disrupted the basal positioning of myoepithelial cells [5, 39]. These experiments suggested that functional desmosomes between adjacent myoepithelial cells and epithelial cells are involved in the formation of acinar-like structures. It remains to be shown whether laminin or desmosomal proteins are sufficient for polarity or whether both are required; this question is under investigation in our laboratory.
Three-dimensional culture method versus rotary culture. The methods shown utilize isolated purified human breast luminal and myoepithelial cells from reduction mammoplasty. In the three-dimensional culture method, coculture of purified luminal and myoepithelial cells in collagen I gel results in the formation of two different types of structures. The majority are (a) a single layer of cells that form acinar structures in which the secretion of laminin-1 by surrounding myoepithelial cells signals to luminal cells to polarize correctly, and the minority are (b) double layer acinar structures that are more reflective of the acinus in vivo. Gudjonsson and coworkers [15] showed that myoepithelial cells were able to induce correct luminal polarity via the synthesis of the basement membrane (BM) component laminin-1. In contrast, in the (c) rotary culture method, purified luminal and myoepithelial cells are grown in suspension. Acinar structures form, albeit at a smaller size compared with the three-dimensional method. Runswick and coworkers [5] showed that blocking desmosome adhesion via blocking peptides inhibited acinar formation.
Several transgenic mouse models have provided further insight into the role played by myoepithelial cells during mammary gland morphogenesis. The cell adhesion receptor P-cadherin is localized to myoepithelial cells; among mice that are homozygous null for P-cadherin, virgin mice exhibit precocious mammary gland development similar to the differentiation that is normally present in early pregnant animals [40]. These findings suggest that myoepithelial expression of P-cadherin may provide an inhibitory signal for luminal cell growth [41]. The parathyroid hormone-related peptide (PTHrP) has been implicated in epithelial–stromal interactions during mammary gland development [42]. In the K14-PTHrP transgenic model, overexpression of the peptide hormone PTHrP in myoepithelial cells inhibits side branching, and ductal elongation is stunted compared with wild-type mice, suggesting that perturbing myoepithelial–stromal interactions affects growth and differentiation of luminal cells [43].
These studies provide insight into specific processes by which myoepithelial cells transmit information for apicobasal polarity and branching morphogenesis; future studies will need to focus on the molecular mechanisms by which these factors interact to establish the acinar structure and the hierarchical nature of their activities.
Paracrine regulator during morphogenesis
Ductal elongation requires the production and organization of new BM, and myoepithelial cells play a key role in these processes as well. Myoepithelial cells synthesize BM components such as collagen IV, laminin-1, laminin-5, and fibronectin that regulate ductal growth [44], and facilitate the sculpting of new BM through the production of matrix metalloproteinases (MMPs), including MMP2 and MMP3 [45]. Myoepithelial cells also express morphogens and growth factors that are activated in a coordinated manner during morphogenesis. Neogenin, a receptor initially identified to act in short and long range neuronal guidance (for review [46]), is expressed by myoepithelial cells and cap cells in terminal end buds (TEBs), a specialized structure at the end of growing ducts [47]. In the mouse, the neogenin knockout is perinatally lethal; however, transplantation studies have shown that mammary glands null for neogenin exhibit altered TEBs that appear disorganized, display breaks in the BM, and contain aberrant subcapsular spaces [47]. It is thought that neogenin may mediate netrin-dependent cell clustering, which is required for the proper formation of the TEB structure [47]. Similarly, the ephrin receptor ephB4 is selectively expressed in myoepithelial cells [48], and mouse mammary tumor virus (MMTV)-ephB4 transgenic mice exhibit defects in mammary gland development with delayed maturation, decreased branching, and decreased alveolar development [49]. Myoepithelial cells also express the heparin-binding growth factor pleiotrophin (also known as HARP), which is active during growth and development [50], and epimorphin, a morphogen that is required for mouse mammary gland branching in three-dimensional culture assays [51]. Over-expression of epimorphin disrupts the organization of the ductal tree in transgenic mice [52]. Furthermore, myoepithelial cells synthesize and secrete basic fibroblast growth factor (bFGF) [53–55] and hepatocyte growth factor (HGF/SF), which function during tubular morphogenesis [56]. (In culture assays HGF is believed to be sufficient to mediate branching [57]; however, we previously showed that it does so only if epimorphin is also expressed [51].) Also, myoepithelial cells may modulate HGF-stimulated branching by expression of activin Ba, a member of the transforming growth factor-β superfamily [58]. Sophisticated branching morphogenesis assays utilizing isolated luminal and myoepithelial cells will be necessary to dissect how these interactions control mammary gland branching.
Myoepithelial cells act in tumor suppression
The majority of breast cancer studies have focused on luminal cells, because these are known to be the source of most carcinomas of the breast (for review [1]). However, progression to carcinoma involves alteration of the entire organized structure of the breast; depending on tumor grade, the changes can include the loss of apicobasal polarity, collapse of the glandular structure, disappearance of normal myoepithelial cells, and disruption of the BM at the epithelial–stromal junction [1]. The mechanisms responsible for the loss of the myoepithelial layer and BM in invasive cancer are unknown. Man and Sang [59] proposed that loss of myoepithelial cells in cancer is due to localized death of these cells; however, this is not proven, and the potential factors responsible for selective cell death are not known. How myoepithelial cells may act to suppress tumor progression in vivo and how these functions are compromised during cancer development remain major unanswered questions.
It is generally believed that myoepithelial cells rarely become malignant (for review [60]). Recently, Angele and coworkers [61] found that human luminal and myoepithelial cells differ in their DNA repair capacity, and this may contribute to the lower rate of transformation in myoepithelial cells. Additionally, when they do undergo transformation, they usually form benign or low-grade neoplasms. Myoepithelial cells express many ECM structural proteins, proteinase inhibitors and angiogenic inhibitors, and accumulate ECM rather than degrade it, which may explain in part why these lesions are not invasive [62, 63].
In addition, myoepithelial cells express a number of type II tumor suppressor genes, defined as factors that affect phenotype through changes in expression rather than through genetic mutation (Table 2) [64, 65]. Barsky and coworkers [62, 66] were the first to use functional assays to show that myoepithelial cells exhibit many antitumorigenic properties, such as the ability to inhibit tumor cell invasion and angiogenesis. Subsequent studies revealed that myoepithelial conditioned media inhibited the growth of breast cancer cell lines and induced a G2/M cell cycle arrest [67]. The ability of myoepithelial cells to inhibit breast cancer cell growth and invasion may in part be attributed to their expression of maspin, a member of the serpin family of serine protease inhibitors. Over-expression of maspin in the breast cancer cell line MDA-MB-435 resulted in inhibition of tumor functions such as growth, angiogenesis, and invasion [68]. In addition, Jones and coworkers [69] showed that myoepithelial cells inhibit invasion through downregulation of MMP expression by tumor cells and fibroblasts. These data suggest that normal myoepithelial cells inhibit tumor cell function through a combined suppression of tumor cell growth, invasion, and angiogenesis.
Do cancer myoepithelial cells have altered function?
The myoepithelial layer appears to remains intact in ductal carcinoma in situ (DCIS); despite this, the myoepithelial cells appear to be aberrant because they differ from normal myoepithelial cells in gene expression, and secrete many chemokines and other factors [70]. This indicates that although myoepithelial cells are present, they no longer send the correct signals to luminal cells. This observation raises the question of whether there are differences between normal myoepithelial cells and those myoepithelial cells that are present in DCIS. Gudjonsson and coworkers [15] found that 20% of carcinomas in which myoepithelial cells were present expressed little or no laminin-1, and that purified cancer myoepithelial cells were unable, for the most part, to 'polarize' luminal cells in three-dimensional collagen assays. These data suggested that cancer myoepithelial cells might be unable to transmit the necessary cues to induce correct luminal cell polarity, at least in part due to their inability to produce laminin-1.
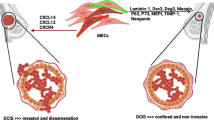
In another study, Allinen and coworkers [70] used SAGE (serial analysis of gene expression) to identify gene expression differences between myoepithelial cells isolated from normal and DCIS samples (Fig. 3). Moreover, those investigators found that cancer myoepithelial cells exhibited the greatest changes in gene expression, and that the chemokine CXCL14 was expressed at higher levels in the DCIS myoepithelial cells than in normal myoepithelial cells. Recently, the chemokine CXCL12/SDF-1 and its receptor CXCR4 were implicated in the induction of tumor cell growth and metastasis [71–73]. Thus, cancer myoepithelial cells, rather than being tumor suppressors, may act to induce growth, migration, and invasion of breast cancer cells, and to undermine the integrity of BM.
DCIS myoepithelial cells exhibit an altered gene expression. In the normal breast myoepithelial cells (MEPs) are located between the luminal cells and the basement membrane. By their location they might act as a barrier to tumor invasion. In ductal carcinoma in situ (DCIS) the myoepithelial layer is still present; however, Allinen and coworkers [70] recently showed that there appears to be molecular differences between MEPs present in normal breast versus DCIS lesions.
Partial myoepithelial differentiation in invasive cancer
Myoepithelial cells and myoepithelial differentiation are largely absent in breast cancer (for review [74]), although there are exceptions to this rule [75–77]. In the microarray analysis performed by Perou and coworkers [78], the 15% (6/40 cases) of tested breast cancer cases that exhibited partial myoepithelial differentiation were also estrogen receptor (ER) negative, and Keese-Adu and colleagues [79] found that 29% (22/77 cases) of tested ER-negative breast cancer samples also exhibited a partial myoepithelial phenotype. These observations suggest a relationship between the loss of ER expression and acquisition of myoepithelial characteristics in breast cancer cells. The expression of the myoepithelial proteins keratin 14, α6β4 integrin, and Dsg 3 in breast cancer cell lines has been shown to correlate with a more aggressive phenotype in cell culture assays [80]. The role played by myoepithelial cells in normal breast as mediators of cell–ECM survival signaling and controllers of morphogenesis may provide insight into why the loss of myoepithelial cells in tumors appears to be linked to expression of myoepithelial characteristics in some breast cancer cells; expression of myoepithelial proteins such as α6β4 integrin may promote tumor cell survival and metastasis in the absence of tumor suppressive functions of normal myoepithelial cells [22].
Conclusion
A key unknown player is the nature of the myoepithelial precursor cell, identification of which may help to define the pathways that stimulate myoepithelial differentiation and how these pathways are disrupted during tumorigenesis. We and others have shown that a bipotential progenitor cell may reside in the luminal cell compartment; in cell culture suprabasal luminal cells (MUC1-/ESA+) are able to generate both luminal and myoepithelial cells [81, 82]. If myoepithelial cells are derived from a bipotential cell, then what pathways stimulate myoepithelial fate? Using the mammosphere culture system, Dontu and coworkers [83] showed that Notch signaling stimulates multipotential progenitor cells to adopt a myoepithelial lineage specific commitment. The Wnt signaling pathway has also been implicated in myoepithelial differentiation. Lie and coworkers [84] found that mammary gland hyperplasias and tumors from Wnt-1 transgenic mice contained a population of cells that expressed progenitor cell markers Keratin-6 and Sca-1 [84]. Interestingly, the Wnt-1 tumors stained positive for both luminal and myoepithelial cell markers, and similar results were found with the MMTV–β-catenin and MMTV–c-myc transgenic mouse models. Loss of heterozygosity for PTEN was detected in both the luminal and myoepithelial cells, suggesting a common origin [84].
Clearly, the function of myoepithelial cells in the breast is more than just contractility, and myoepithelial cells are more than a fence between the milk-producing luminal cells and the surrounding stroma. It is clear that much remains to be learned about the physiological role of these cells in the normal breast and the functional differences between normal and cancer myoepithelial cells.
Abbreviations
- BM:
-
basement membrane
- DCIS:
-
ductal carcinoma in situ
- Dsc:
-
desmocollin
- Dsg:
-
desmoglein
- ECM:
-
extracellular matrix
- ER:
-
estrogen receptor
- HGF:
-
hepatocyte growth factor
- MMP:
-
matrix metalloproteinase
- MMTV:
-
mouse mammary tumor virus
- PTHrP:
-
parathyroid hormone-related peptide
- TEB:
-
terminal end bud.
References
Ronnov-Jessen L, Petersen OW, Bissell MJ: Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996, 76: 69-125.
Medina D: The mammary gland: a unique organ for the study of development and tumorigenesis. J Mammary Gland Biol Neoplasia. 1996, 1: 5-19.
Emerman JT, Vogl AW: Cell size and shape changes in the myoepithelium of the mammary gland during differentiation. Anat Rec. 1986, 216: 405-415. 10.1002/ar.1092160310.
Radnor CJ: Myoepithelial cell differentiation in rat mammary glands. J Anat. 1972, 111: 381-398.
Runswick SK, O'Hare MJ, Jones L, Streuli CH, Garrod DR: Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001, 3: 823-830. 10.1038/ncb0901-823.
Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ: Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989, 105: 223-235.
Franke WW, Schmid E, Freudenstein C, Appelhans B, Osborn M, Weber K, Keenan TW: Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980, 84: 633-654. 10.1083/jcb.84.3.633.
Page MJ, Amess B, Townsend RR, Parekh R, Herath A, Brusten L, Zvelebil MJ, Stein RC, Waterfield MD, Davies SC, et al: Proteomic definition of normal human luminal and myoepithelial breast cells purified from reduction mammoplasties. Proc Natl Acad Sci USA. 1999, 96: 12589-12594. 10.1073/pnas.96.22.12589.
Ahmed A: The myoepithelium in human breast carcinoma. J Pathol. 1974, 113: 129-135. 10.1002/path.1711130208.
Koukoulis GK, Virtanen I, Korhonen M, Laitinen L, Quaranta V, Gould VE: Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991, 139: 787-799.
Bergstraesser LM, Srinivasan G, Jones JC, Stahl S, Weitzman SA: Expression of hemidesmosomes and component proteins is lost by invasive breast cancer cells. Am J Pathol. 1995, 147: 1823-1839.
Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK: Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J Cell Biol. 1973, 56: 797-818. 10.1083/jcb.56.3.797.
Hamperl H: The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970, 53: 161-220.
Monaghan P, Clarke C, Perusinghe NP, Moss DW, Chen XY, Evans WH: Gap junction distribution and connexin expression in human breast. Exp Cell Res. 1996, 223: 29-38. 10.1006/excr.1996.0055.
Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW: Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002, 115: 39-50.
Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, Dale T, Gusterson B, Kamalati T: HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development. 1995, 121: 2897-2908.
Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH: The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res. 1997, 3: 1949-1958.
Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ: Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992, 89: 9064-9068.
Coleman S, Silberstein GB, Daniel CW: Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988, 127: 304-315. 10.1016/0012-1606(88)90317-X.
Mork C, van Deurs B, Petersen OW: Regulation of vimentin expression in cultured human mammary epithelial cells. Differentiation. 1990, 43: 146-156.
Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJ, Sager R: Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996, 93: 11669-11674. 10.1073/pnas.93.21.11669.
Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ: beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002, 2: 205-216. 10.1016/S1535-6108(02)00125-3.
Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ: A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002, 62: 7102-7109.
Ekblom O, Lonai P, Talts JF: Expression and biological role of laminin-1. Matrix Biol. 2003, 22: 35-47. 10.1016/S0945-053X(03)00015-5.
Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D: Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999, 144: 151-160. 10.1083/jcb.144.1.151.
Miner JH, Li C, Mudd JL, Go G, Sutherland AE: Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004, 131: 2247-2256. 10.1242/dev.01112.
Scheele S, Falk M, Franzen A, Ellin F, Ferletta M, Lonaio P, Andersson B, Timpl R, Forsberg E, Ekblom P: Laminin {alpha}1 globular domains 4–5 induce fetal development but are not vital for embryonic basement membrane assembly. Proc Natl Acad Sci USA. 2005, 102: 1502-1506. 10.1073/pnas.0405095102.
Miner JH, Yurchenco PD: Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004, 20: 255-284. 10.1146/annurev.cellbio.20.010403.094555.
Sorokin LM, Conzelmann S, Ekblom P, Battaglia C, Aumailley M, Timpl R: Monoclonal antibodies against laminin A chain fragment E3 and their effects on binding to cells and proteoglycan and on kidney development. Exp Cell Res. 1992, 201: 137-144. 10.1016/0014-4827(92)90357-E.
Simon-Assmann P, Kedinger M, De Arcangelis A, Rousseau V, Simo P: Extracellular matrix components in intestinal development. Experientia. 1995, 51: 883-900.
Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P: Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995, 129: 521-534. 10.1083/jcb.129.2.521.
Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD: The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003, 4: 613-624. 10.1016/S1534-5807(03)00128-X.
Streuli CH, Bailey N, Bissell MJ: Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991, 115: 1383-1395. 10.1083/jcb.115.5.1383.
Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ: Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995, 129: 591-603. 10.1083/jcb.129.3.591.
Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ: Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Mol Biol Cell. 1999, 10: 2817-2828.
Colognato H, Yurchenco PD: Form and function: the laminin family of heterotrimers. Dev Dyn. 2000, 218: 213-234. 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R.
O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE: Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001, 3: 831-838. 10.1038/ncb0901-831.
Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ: Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995, 108: 1945-1957.
Bissell MJ, Bilder D: Polarity determination in breast tissue: desmosomal adhesion, myoepithelial cells, and laminin 1. Breast Cancer Res. 2003, 5: 117-119. 10.1186/bcr579.
Daniel CW, Strickland P, Friedmann Y: Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev Biol. 1995, 169: 511-519. 10.1006/dbio.1995.1165.
Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO: Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997, 139: 1025-1032. 10.1083/jcb.139.4.1025.
Wiseman BS, Werb Z: Stromal effects on mammary gland development and breast cancer. Science. 2002, 296: 1046-1049. 10.1126/science.1067431.
Wysolmerski JJ, McCaughern-Carucci JF, Daifotis AG, Broadus AE, Philbrick WM: Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development. 1995, 121: 3539-3547.
Warburton MJ, Mitchell D, Ormerod EJ, Rudland P: Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982, 30: 667-676.
Dickson SR, Warburton MJ: Enhanced synthesis of gelatinase and stromelysin by myoepithelial cells during involution of the rat mammary gland. J Histochem Cytochem. 1992, 40: 697-703.
Tessier-Lavigne M, Goodman CS: The molecular biology of axon guidance. Science. 1996, 274: 1123-1133. 10.1126/science.274.5290.1123.
Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L: Netrin-1/ neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003, 4: 371-382. 10.1016/S1534-5807(03)00054-6.
Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A: Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci. 1998, 111: 2741-2751.
Munarini N, Jager R, Abderhalden S, Zuercher G, Rohrbach V, Loercher S, Pfanner-Meyer B, Andres AC, Ziemiecki A: Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J Cell Sci. 2002, 115: 25-37.
Ledoux D, Caruelle D, Sabourin JC, Liu J, Crepin M, Barritault D, Courty J: Cellular distribution of the angiogenic factor heparin affin regulatory peptide (HARP) mRNA and protein in the human mammary gland. J Histochem Cytochem. 1997, 45: 1239-1245.
Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ: Epimorphin functions as a key morphoregulator for mammary epithelial cells. J Cell Biol. 1998, 140: 159-169. 10.1083/jcb.140.1.159.
Hirai Y, Radisky D, Boudreau R, Simian M, Stevens ME, Oka Y, Takebe K, Niwa S, Bissell MJ: Epimorphin mediates mammary luminal morphogenesis through control of C/EBPbeta. J Cell Biol. 2001, 153: 785-794. 10.1083/jcb.153.4.785.
Rudland PS, Platt-Higgins AM, Wilkinson MC, Fernig DG: Immunocytochemical identification of basic fibroblast growth factor in the developing rat mammary gland: variations in location are dependent on glandular structure and differentiation. J Histochem Cytochem. 1993, 41: 887-898.
Gomm JJ, Browne PJ, Coope RC, Bansal GS, Yiangou C, Johnston CL, Mason R, Coombes RC: A paracrine role for myoepithelial cell-derived FGF2 in the normal human breast. Exp Cell Res. 1997, 234: 165-173. 10.1006/excr.1997.3593.
Gomm JJ, Smith J, Ryall GK, Baillie R, Turnbull L, Coombes RC: Localization of basic fibroblast growth factor and transforming growth factor beta 1 in the human mammary gland. Cancer Res. 1991, 51: 4685-4692.
Rosario M, Birchmeier W: How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003, 13: 328-335. 10.1016/S0962-8924(03)00104-1.
Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ: The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001, 128: 3117-3131.
Liu QY, Niranjan B, Gomes P, Gomm JJ, Davies D, Coombes RC, Buluwela L: Inhibitory effects of activin on the growth and morpholgenesis of primary and transformed mammary epithelial cells. Cancer Res. 1996, 56: 1155-1163.
Man YG, Sang QX: The significance of focal myoepithelial cell layer disruptions in human breast tumor invasion: a paradigm shift from the 'protease-centered' hypothesis. Exp Cell Res. 2004, 301: 103-118. 10.1016/j.yexcr.2004.08.037.
Lakhani SR, O'Hare MJ: The mammary myoepithelial cell: Cinderella or ugly sister?. Breast Cancer Res. 2001, 3: 1-4. 10.1186/bcr260.
Angele S, Jones C, Reis Filho JS, Fulford LG, Treilleux I, Lakhani SR, Hall J: Expression of ATM, p53, and the MRE11-Rad50-NBS1 complex in myoepithelial cells from benign and malignant proliferations of the breast. J Clin Pathol. 2004, 57: 1179-1184. 10.1136/jcp.2004.017434.
Sternlicht MD, Safarians S, Rivera SP, Barsky SH: Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest. 1996, 74: 781-796.
Barsky SH: Myoepithelial mRNA expression profiling reveals a common tumor-suppressor phenotype. Exp Mol Pathol. 2003, 74: 113-122. 10.1016/S0014-4800(03)00011-X.
Sager R: Expression genetics in cancer: shifting the focus from DNA to RNA. Proc Natl Acad Sci USA. 1997, 94: 952-955. 10.1073/pnas.94.3.952.
Bissell MJ, Radisky D: Putting tumours in context. Nat Rev Cancer. 2001, 1: 46-54. 10.1038/35094059.
Nguyen M, Lee MC, Wang JL, Tomlinson JS, Shao ZM, Alpaugh ML, Barsky SH: The human myoepithelial cell displays a multifaceted anti-angiogenic phenotype. Oncogene. 2000, 19: 3449-3459. 10.1038/sj.onc.1203677.
Shao ZM, Nguyen M, Alpaugh ML, O'Connell JT, Barsky SH: The human myoepithelial cell exerts antiproliferative effects on breast carcinoma cells characterized by p21WAF1/CIP1 induction, G2/M arrest, and apoptosis. Exp Cell Res. 1998, 241: 394-403. 10.1006/excr.1998.4066.
Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R: Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994, 263: 526-529.
Jones JL, Shaw JA, Pringle JH, Walker RA: Primary breastmyoepithelial cells exert an invasion-suppressor effect on breast cancer cells via paracrine down-regulation of MMP expression in fibroblasts and tumour cells. J Pathol. 2003, 201: 562-572. 10.1002/path.1483.
Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al: Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004, 6: 17-32. 10.1016/j.ccr.2004.06.010.
Hall JM, Korach KS: Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003, 17: 792-803. 10.1210/me.2002-0438.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al: Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001, 410: 50-56. 10.1038/35065016.
Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, PiwnicaWorms D, Luker GD: CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004, 64: 8604-8612.
Rudland PS, Fernig DG, Smith JA: Growth factors and their receptors in neoplastic mammary glands. Biomed Pharmacother. 1995, 49: 389-399. 10.1016/0753-3322(96)82676-X.
Nagle RB, Bocker W, Davis JR, Heid HW, Kaufmann M, Lucas DO, Jarasch ED: Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem. 1986, 34: 869-881.
Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS: Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982, 42: 4763-4770.
Guelstein VI, Tchypysheva TA, Ermilova VD, Litvinova LV, Troyanovsky SM, Bannikov GA: Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer. 1988, 42: 147-153.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al: Molecular portraits of human breast tumours. Nature. 2000, 406: 747-752. 10.1038/35021093.
Kesse-Adu R, Shousha S: Myoepithelial markers are expressed in at least 29% of oestrogen receptor negative invasive breast carcinoma. Mod Pathol. 2004, 17: 646-652. 10.1038/modpathol.3800103.
Gordon LA, Mulligan KT, Maxwell-Jones H, Adams M, Walker RA, Jones JL: Breast cell invasive potential relates to the myoepithelial phenotype. Int J Cancer. 2003, 106: 8-16. 10.1002/ijc.11172.
Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW: Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol. 1999, 206: 88-99. 10.1006/dbio.1998.9133.
Stingl J, Eaves CJ, Zandieh I, Emerman JT: Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001, 67: 93-109. 10.1023/A:1010615124301.
Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS: Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004, 6: R605-R615. 10.1186/bcr920.
Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al: Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003, 100: 15853-15858. 10.1073/pnas.2136825100.
Dairkee SH, Blayney C, Smith HS, Hackett AJ: Monoclonal antibody that defines human myoepithelium. Proc Natl Acad Sci USA. 1985, 82: 7409-7413.
Ronnov-Jessen L, Van Deurs B, Nielsen M, Petersen OW: Identification, paracrine generation, and possible function of human breast carcinoma myofibroblasts in culture. In Vitro Cell Dev Biol. 1992, 28A: 273-283.
Moll R: Cytokeratins as markers of differentiation. Expression profiles in epithelia and epithelial tumors [in German]. Veroff Pathol. 1993, 142: 1-197.
Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G: A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986, 103: 2787-2796. 10.1083/jcb.103.6.2787.
Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE: Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA. 1993, 90: 999-1003.
Gusterson BA, Monaghan P, Mahendran R, Ellis J, O'Hare MJ: Identification of myoepithelial cells in human and rat breasts by anti-common acute lymphoblastic leukemia antigen antibody A12. J Natl Cancer Inst. 1986, 77: 343-349.
Rasbridge SA, Gillett CE, Sampson SA, Walsh FS, Millis RR: Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol. 1993, 169: 245-250. 10.1002/path.1711690211.
Natali PG, Nicotra MR, Botti C, Mottolese M, Bigotti A, Segatto O: Changes in expression of alpha 6/beta 4 integrin heterodimer in primary and metastatic breast cancer. Br J Cancer. 1992, 66: 318-322.
Koukoulis GK, Howeedy AA, Korhonen M, Virtanen I, Gould VE: Distribution of tenascin, cellular fibronectins and integrins in the normal, hyperplastic and neoplastic breast. J Submicrosc Cytol Pathol. 1993, 25: 285-295.
Wilgenbus KK, Kirkpatrick CJ, Knuechel R, Willecke K, Traub O: Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. Int J Cancer. 1992, 51: 522-529.
Rudland PS, Hughes CM, Ferns SA, Warburton MJ: Characterization of human mammary cell types in primary culture: immunofluorescent and immunocytochemical indicators of cellular heterogeneity. In Vitro Cell Dev Biol. 1989, 25: 23-36.
Schmid KW, Ellis IO, Gee JM, Darke BM, Lees WE, Kay J, Cryer A, Stark JM, Hittmair A, Ofner D, et al: Presence and possible significance of immunocytochemically demonstrable metallothionein over-expression in primary invasive ductal carcinoma of the breast. Virchows Arch A Pathol Anat Histopathol. 1993, 422: 153-159. 10.1007/BF01607167.
Ronnov-Jessen L, Petersen OW: A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol. 1996, 134: 67-80. 10.1083/jcb.134.1.67.
Okamoto-Inoue M, Kamada S, Kimura G, Taniguchi S: The induction of smooth muscle alpha actin in a transformed rat cell line suppresses malignant properties in vitro and in vivo. Cancer Lett. 1999, 142: 173-178. 10.1016/S0304-3835(99)00150-0.
Zajchowski DA, Band V, Trask DK, Kling D, Connolly JL, Sager R: Suppression of tumor-forming ability and related traits in MCF-7 human breast cancer cells by fusion with immortal mammary epithelial cells. Proc Natl Acad Sci USA. 1990, 87: 2314-2318.
Sager R, Anisowicz A, Neveu M, Liang P, Sotiropoulou G: Identification by differential display of alpha 6 integrin as a candidate tumor suppressor gene. FASEB J. 1993, 7: 964-970.
Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE: Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998, 16: 1391-1397. 10.1038/sj.onc.1201661.
Hirschi KK, Xu CE, Tsukamoto T, Sager R: Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996, 7: 861-870.
Bani D, Riva A, Bigazzi M, Bani Sacchi T: Differentiation of breast cancer cells in vitro is promoted by the concurrent influence of myoepithelial cells and relaxin. Br J Cancer. 1994, 70: 900-904.
Acknowledgements
We thank DC Radisky for critical reading of the manuscript and insightful comments. The work from the authors' laboratory is supported by funds from the United States Department of Energy, Office of Biological and Environmental Research (DE AC03 76SF00098, MJB), the National Cancer Institute (CA 64786 to MJB and CA 57621 to Zena Werb and MJB) and Innovator Award from the United States Department of Defense Breast Cancer Research Program (DAMD17-02-1-0438) to MJB and by a NCI fellowship to MCA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Adriance, M.C., Inman, J.L., Petersen, O.W. et al. Myoepithelial cells: good fences make good neighbors. Breast Cancer Res 7, 190 (2005). https://doi.org/10.1186/bcr1286
Published:
DOI: https://doi.org/10.1186/bcr1286