Abstract
A new Zn(II) coordination polymer based on oxalic acid and 1,4-bis(imidazol-1-ylmethyl)benzene (bix), namely, [Zn(C2O4)(bix)]n (1), has been successfully synthesized under hydrothermal conditions. Its structure has been determined by single crystal X-ray diffraction analysis, elemental analyses and IR spectroscopy. Compound 1 shows a two-dimensional (2D) layer structure. The intermolecular C–H···O interactions extend the compound 1 into 3D supramolecular architectures and play an important role in stabilizing compound 1. In addition, the luminescent property of the compound has also been investigated in solid state at room temperature.
Similar content being viewed by others
1 Introduction
Researches on the metal-directed extended networks have drawn great attention owing not only to their intriguing structural motifs but also to their potential applications in catalysis, medicine, hostguest chemistry and molecular-based magnetic materials [1–5]. In this field, the metals often have different valences, making a number of building blocks to fulfill special needs. Many important properties of coordination polymers depend largely on their structures and topology. Therefore, the selection of special inorganic and organic building blocks is the key to the construction of a desired framework [6]. In this respect, the oxalate ligand is proved to be a good candidate due to its various bridging abilities and strong coordination tendency with transition metals to form 2- and 3-D moderately robust networks exhibiting tunable ferro- or antiferro-magnetic exchanges [7–9]. On the other hand, the introduction of bi- or multi-dentate ligands containing N- or O-donors to the metal-oxalate system may lead to new structural evolution since the binding of these ligands to metal centers may adjust the dimensionality of metalorganic coordination polymers [10, 11]. Among the organic N-donors, 1,4-bis(imidazol-1-ylmethyl)-benzene (bix) is an excellent ligand for the construction of novel metal–organic coordination frameworks because of its two donor sites [12]. In this paper, we report a new Zn(II) coordination polymer [Zn(C2O4)(bix)]n 1, in which the 1D chains are held together via bix ligands to form a two-dimensional layer structure. Such 2D structure geometry is reported scarcely.
2 Experimental Section
2.1 General Procedures
All reagents were purchased commercially and used without further purification. Elemental analyses (C, H and N) were measured on a Perkin-Elmer 2400 CHN Elemental Analyzer. IR spectrum was recorded in the range of 4,000–400 cm−1 on an Alpha Centaurt FT/IR Spectrophotometer using a KBr pellet. The fluorescent studies were carried out on a computer-controlled JY Fluoro-Max-3 spectrometer at room temperature.
2.2 Synthesis of [Zn(C2O4)(bix)]n (1)
The title compound was prepared from a mixture of Zn(OAc)2·2H2O (0.044 g, 0.2 mmol), H2C2O4·2H2O (0.050 g, 0.4 mmol), bix (0.048 g, 0.2 mmol) and H2O (18 mL) in a 30 mL Teflon-lined stainless steel vessel, and then the vessel was sealed and heated at 150 °C for 7 days. After the reaction mixture was slowly cooled down to room temperature at the rate of 5 °C/h, pale yellow block crystals of compound 1 were obtained. Yield: 52 %. Anal. Calcd. For C16H14N4O4Zn: C, 49.06; H, 3.60; N, 14.30. Found C, 49.01; H, 3.58; N, 14.27. IR (cm−1): 3,103(w), 1,671(vs), 1,609(vs), 1,515(m), 1,313(m), 1,231(m), 1,112(m), 940(m), 796(m), 720(w), 659(w), 492(w).
2.3 X-ray Crystallographic Study of Compound (1)
Single-crystal X-ray diffraction data for 1 was recorded on a Bruker Smart Apex II CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 293 K. The structure was solved with the direct method of SHELXS-97 and refined with full-matrix least-squares techniques using the SHELXL-97 program [13, 14]. The non-hydrogen atoms of the complexes were refined with anisotropic temperature parameters. The hydrogen atoms attached to carbons were generated geometrically. Crystallographic parameters and the data collection statistics for structure 1 are given in Table 1. Selected bond lengths and bond angles are listed in Table 2. Further crystallographic parameters have been deposited with the Cambridge Crystallographic Data Centre (no. 874947; deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk/data_request/cif).
3 Results and Discussion
3.1 Syntheses and General Characterization
The new d10 metal complex reported here was simply synthesized under hydrothermal reaction condition. Complex 1 was controlled by the amount of triethylamine. In the reaction the molar ratio of Zn(OAc)2·2H2O with bix, H2C2O4·2H2O was kept at 1:1:2. However, the resulting products show small crystals when heating at 120 °C, yield: 35 %; when heating at 150 °C, block crystals suitable for the determination of X-ray were obtained, yield: 52 %.
3.2 IR Spectrum
The COO− is coordinated with its asymmetric and symmetric stretching appearing at 1,608 cm−1 (v(OCO)asym) and 1,428 cm−1 (v(OCO)sym) [15], respectively. The Δν (ν(OCO)asym–ν(OCO)sym) is 180 cm−1 (<200), showing the presence of bidentate linkage of carboxylates in the dianions. Thus the carboxylates coordinate to the metal as bidentate ligands via the carboxylate groups [16]. The absence of characteristic bands around 1,700 cm−1 in compound 1 attributed to the protonated carboxylic group indicates the complete deprotonation of H2C2O4 ligand upon reaction with Zn ions [17]. In addition, X-ray diffraction analysis further indicates the bidentate coordination manners of carboxylate groups and the deprotonation of H2C2O4 ligands.
3.3 Description of the Structure
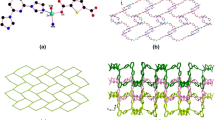
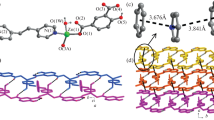
A single-crystal X-ray diffraction study reveals that compound 1 crystallizes in the monoclinic space group C2/c and features a two-dimensional (2D) layer-like structure. The asymmetric unit of 1 contains one six-coordinated Zn(II) atom, one C2O4 2− ligand and one bix ligand, as shown in Fig. 1. Each Zn(II) ion is six coordinated with distorted octahedral coordination geometry defined by two nitrogen donors (N(1) and N(1)#1) from two different bix molecules and four carboxylate oxygen atoms (O(1), O(1)#1, O(2)#2, O(2)#3) from two different C2O4 2− ligands. Three carboxylate oxygen (O(1), O(1)#1, O(2)#3) and one nitrogen (N(1)#1) atom define an equatorial plane, while the axial coordination sites are occupied by carboxylate oxygen (O(2)#2) and nitrogen (N(1)) atom. The bond distances of Zn–O in compound 1 fall in the 2.089(3)–2.148(3) Å range, and Zn–N bond length is 2.115(3)–2.115(4) Å, which are in the normal range and the coordination angles around Zn atom are in the range 78.73(10)–168.78(12)º. The completely deprotonated C2O4 2− ligands display one kind of coordination mode, namely bidentate bridging mode, and the bix ligand adopts trans-conformation bridging mode with a dihedral angle between the two imidazole rings of 0º.
The Zn(II) centers forms a type of 4O+2N mixed neutrality complex, and are interconnected by the bridging oxalate ligands to generate an infinite {Zn2(C2O4)2}∞ chain along the a axis. The Zn···Zn separation through the oxalate bridges is 5.667 Ǻ, the neighboring 1D {Zn2(C2O4)2}∞ infinite chains are linked via bix ligands to develop into 2D layer framework (Fig. 2), which is similar with our reported paper [18]. In [18], the structure of complex ([Zn(C2O4)(1,3-bix)]n) is 2D network with (4,4) topology. From the packing diagram, we can see that the C2O4 2− and bix ligands are also linked by means of C–H···O hydrogen-bonding interactions (C1···O1 = 3.401(8) Å) which are known to be important in the synthesis of supramolecular architectures [19, 20] and undoubtedly play an important role in stabilizing compound 1 (Fig. 3).
To investigate whether the analyzed crystal structure is truly representative of the bulk materials, X-ray powder diffraction (PXRD) technology has been performed for the complex at room temperature (Fig. 4). The main peak positions observed are in good agreement with the simulated ones. Although minor differences can be found in the positions, widths, and intensities of some peaks, it still can be considered that the bulk synthesized materials and the analyzed crystal are homogeneous. The differences may be due to the preferred orientation of the powder samples [21, 22].
3.4 Luminescent Properties
Luminescence property is very important in photochemistry and photophysics [23, 24]. So in this study, the solid-state photoluminescence spectra of 1 (Fig. 5), free H2C2O4·2H2O and bix ligands were investigated at room temperature. Excited by 355 nm, coordination polymer 1 gives wide yellow emission with the maximum peak at 599 nm plus shoulder peak at 564 nm. The main emission peak of ligand bix is at 508 nm. However, no obvious emission bands are observed for the free H2C2O4 ligand in the range of 400–800 nm under the same experimental conditions. The significant phenomenon of the fluorescenc emission of 1 here could be tentatively assigned to the ligand-to-metal charge transfer (LMCT) [25]. For possesses strong fluorescent intensity, it appears to be good candidates for novel hybrid inorganic–organic photoactive materials.
4 Theoretical Calculations
All calculations in this work were carried out with the Gaussian 09 program [26]. The parameters of the molecular structure for calculation were all from the experimental data of the complex. Natural bond orbital (NBO) analysis was performed by density functional theory (DFT) [27] with the PBE0 [28, 29] hybrid functional and the LANL2DZ basis set [30].
The selected natural atomic charges and natural electron configuration for the complex is shown in Table 3. It is indicated that the electronic configurations of Zn(II) ion, N and O atoms are 4s 0.303d 9.984p 0.44, 2s 1.70–1.722p 4.85–5.013p 0.01 and 2s 1.382p 4.203p 0.02, respectively. Based on the above results, one can conclude that the Zn(II) ion coordination with N and O atoms is mainly on 3d, 4s, and 4p orbitals. N atoms form coordination bonds with Zn(II) ion using 2s and 2p orbitals. All O atoms supply electrons of 2s and 2p to Zn(II) ion and form the coordination bonds. Therefore, the Zn(II) ion obtained some electrons from two N atoms of bix ligand, four O atoms of C2O4 2− ligand. Thus, according to valence-bond theory the atomic net charge distribution in the complex shows the obvious covalent interaction between the coordinated atoms and Zn(II) ion. As can be seen from the Fig. 6, the HOMO mainly consists of Zn(II) ion and ligand and the LUMO is mainly composed of Zn(II) ion and ligand.
References
S. Leininger, B. Olenyuk, P.J. Stang, Chem. Rev. 100, 853 (2000)
P.J. Hagrman, D. Hagrman, J. Zubieta, Angew. Chem. Int. Ed. 38, 2638 (1999)
X.M. Zhang, M.L. Tong, X.M. Chen, Angew. Chem. Int. Ed. 41, 1029 (2002)
S.H. Feng, R.R. Xu, Acc. Chem. Res. J. 34, 239 (2001)
W.S. You, E.B. Wang, Y. Xu et al., Inorg. Chem. 40, 5468 (2001)
X.M. Li, Q.W. Wang, D. Li et al., Chin. J. Struct. Chem. 11, 1339 (2007)
M. Du, Y.M. Guo, X.H. Bu, Inorg. Chim. Acta 335, 136 (2002)
S. Pérez-Yáňez, O. Castillo, J. Cepeda et al., Inorg. Chim. Acta 365, 211 (2011)
L.M. Zheng, X. Fang, K.H. Li et al., J. Chem. Soc. Dalton Trans. 2311 (1999)
P.J. Hagrman, J. Zubieta, Inorg. Chem. 39, 3252 (2000)
Y.G. Li, E.B. Wang, H. Zhang et al., J. Solid State Chem. 163, 10 (2002)
Z.L. Wang, M.X. Li, J.W. Zhao et al., Chin. J. Struct. Chem. 5, 654 (2011)
G.M. Sheldrick, SHELXS-97, Programs for X-ray Crystal Structure Solution (University of Göttingen, Göttingen, 1997)
G.M. Sheldrick, SHELXL-97, Programs for X-ray Crystal Structure Refinement (University of Göttingen, Göttingen, 1997)
M. Devereux, D.O. Shea, A. Kellett et al., Inorg. Biochem. 101, 881 (2007)
L.J. Farrugia, X.A. Wing, Windows Program for Crystal Structure Analysis (University of Glasgow, Glasgow, 1988)
Z.Y. Fu, X.T. Wu, J.C. Dai et al., Eur. J. Inorg. Chem. 2002, 2730 (2002)
P.Y. Zhan, J.Y. Ji, Y.L. Niu et al., Chin. J. Inorg. Chem. 2, 424 (2013)
M. Yuan, Y.G. Li, E.B. Wang et al., J. Chem. Soc. Dalton Trans. 2916 (2002)
Z.B. Han, E.B. Wang, G.Y. Luan et al., J. Mater. Chem. 12, 1169 (2002)
A. Gilbert, J. Baggott, Essentials of Molecular Photochemistry (CRC Press, Boca Raton, 1991)
Z. Han, Y. He, C. Ge, J. Ribas, L. Xu, Dalton Trans. 3020 (2007)
S. Mizukami, H. Houjou, K. Sugaya et al., Chem. Mater. 17, 50 (2005)
C.W. Tang, S.A. Vanslyke, Appl. Phys. Lett. 51, 913 (1987)
Y.X. Chi, S.Y. Niu, J. Jin et al., Z. Anorg. Allg. Chem. 633, 1277 (2007)
M.J. Frisch, G.W. Trucks, H.B. Schlegel et al., Gaussian 09 (Gaussian Inc., Wallingford, 2009)
R.G. Parr, W. Yang, Density Functional Theory of Atoms and Molecules (Oxford University Press, Oxford, 1989)
M. Ernzerhof, G.E. Scuseria, J. Chem. Phys. 110, 5029 (1999)
C. Adamo, V.J. Barone, Chem. Phys. 110, 6158 (1999)
T.H. Dunning, P.J. Hay Jr, in Modern Theoretical Chemistry, ed. by H.F. Schaefer III (Plenum, New York, 1976), p. 1
Acknowledgments
This work was supported by the Science and Technology Development Project of Jilin Provincial Science & Technology Department (201205080) and the Science and Technology Research Projects of the Education Department of Jilin Province (2012.358). Program supports from State Key Laboratory of Theoretical and Computational Chemistry of Jilin University are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wang, QW., Qi, XF., Li, XM. et al. Synthesis, Crystal Structure and Luminescent Property of a New Two-Dimensional Zn(II) Coordination Polymer Based on Oxalic Acid and 1,4-Bis(imidazol-1-ylmethyl)-benzene. J Inorg Organomet Polym 23, 1313–1317 (2013). https://doi.org/10.1007/s10904-013-9924-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-013-9924-8