Abstract
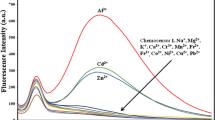
This paper reports development of an iron sensor, 2-(3H-pyrrolo[2,3-c]quinolin-4-yl)aniline (APQ). The fluorophore facilitates micromolar detection of Fe3+/Fe2+ in the presence of various cations, including well-known interfering cations Co2+and Cu2+ by the process of fluorescence quenching.

Graphical Abstract






Similar content being viewed by others
References
Fernandez-Real JM, McClain D, Manco M (2015) Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of Type 2 diabetes. Diabetes Care 38:2169–2176
Rathnasamy G, Ling E-A, Kaur C (2013) Consequences of iron accumulation in microglia and its implications in neuropathological conditions. CNS Neurol Disord Drug Targets 12:785–798
Lushchak V, Semchyshyn H (eds) (2012) Oxidative stress: molecular mechanisms and biological effects. Intech. https://doi.org/10.5772/2333
Youdim MBH (2008) Brain iron deficiency and excess; cognitive impairment and neurodegenration with involvement of striatum and hippocampus. Neurotox Res 14:45–56
Ohashi A, Ito H, Kanai C, Imura H, Ohashi K (2005) Cloud point extraction of iron(III) and vanadium(V) using 8-quinolinol derivatives and Triton X-100 and determination of 10moldm level iron(III) in riverine water reference by a graphite furnace atomic absorption spectroscopy. Talanta 65:525–530
Lunvongsa S, Oshima M, Motomizu S (2006) Determination of total and dissolved amount of iron in water samples using catalytic spectrophotometric flow injection analysis. Talanta 68:969–973
Gomes DMC, Segundo MA, Lima JLFC, Rangel AOSS (2005) Spectrophotometric determination of iron and boron in soil extracts using a multi-syringe flow injection system. Talanta 66:703–711
Tesfaldet ZO, van Staden JF, Stefan RI (2004) Sequential injection spectrophotometric determination of iron as Fe(II) in multi-vitamin preparations using 1,10-phenanthroline as complexing agent*1. Talanta 64:1189–1195
Yokoi K, Van den Berg CMG (1992) The determination of iron in seawater using catalytic cathodic stripping voltammetry. Electroanalysis 4:65–69
Bobrowski A, Nowak K, Zarebski J (2005) Application of a bismuth film electrode to the voltammetric determination of trace iron using a Fe(III)–TEA–BrO3− catalytic system. Anal Bioanal Chem 382:1691–1697
Elrod VA, Johnson KS, Coale KH (1991) Determination of subnanomolar levels of iron(II) and total dissolved iron in seawater by flow injection and analysis with chemiluminescence detection. Anal Chem 63:893–898
Qin W, Zhang ZJ, Zhang CJ (1998) Chemiluminescence flow sensor with immobilized reagents for the determination of iron(III). Microchim Acta 129:97–101
Kimura M, Tsunenaga M, Takami S, Ohbayashi Y (2005) Development of Functional Imidazole Derivatives: A Potential Chemiluminescent Chemosensor. Bull Chem Soc Jpn 78:929–931
Kikkeri R, Traboulsi H, Humbert N, Gumienna-Kontecka E, Arad-Yellin R, Melman G, Elhabiri M, Albrecht-Gary AM, Shanzer A (2007) Toward Iron Sensors: Bioinspired Tripods Based on Fluorescent Phenol-oxazoline Coordination Sites. Inorg Chem 46:2485–2497
Sahoo SK, Sharma D, Bera RK, Crisponi G, Callan JF (2012) Iron(III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 41:7195–7227
Lan L, Niu Q, Guo Z, Liu H, Li T (2017) Highly sensitive and fast responsive “turn-on” fluorescent sensor for selectively sensing Fe 3+ and Hg 2+ in aqueous media based on an oligothiophene derivative and its application in real water samples. Sensors Actuators B Chem 244:500–508
Hua C, Zheng H, Zhang K, Xin M, Gao J, Li Y (2016) A novel turn off fluorescent sensor for Fe(III) and pH environment based on coumarin derivatives: the fluorescence characteristics and theoretical study. Tetrahedron 72:8365–8372
Zhao B, Liu T, Fang Y, Wang L, Song B, Deng Q (2016) A novel colorimetric chemosensor based on quinoline for the sequential detection of Fe3+ and PPi in aqueous solution. Tetrahedron Lett 58:1025–1029
Joshi S, Kumari S, Bhattacharjee R, Sarmah A, Sakhuja R, Pant DD (2015) Experimental and theoretical study: determination of dipole moment of synthesized coumarin–triazole derivatives and application as turn off fluorescence sensor: High sensitivity for iron(III) ions. Sensors Actuators B Chem 220:1266–1278
Sharma D, Kuba A, Thomas R, Kumar R, Choi H-J, Sahoo SK (2016) An aqueous friendly chemosensor derived from vitamin B6 cofactor for colorimetric sensing of Cu2+ and fluorescent turn-off sensing of Fe3+. Spectrochim Acta A 153:393–396
Maity S, Kundu A, Pramanik A (2015) Synthesis of biologically important, fluorescence active 5-hydroxy benzo[g]indoles through four-component domino condensations and their fluorescence “Turn-off” sensing of Fe(iii) ions. RSC Adv 5:52852–52865
Wang W, Wei J, Liu H, Liu Q, Gao Y (2017) A novel colorimetric chemosensor based on quinoline for the sequential detection of Fe3+ and PPi in aqueous solution. Tetrahedron Lett 58:1025–1029
Mukherjee S, Talukdar S (2016) A reversible pyrene-based turn-on luminescent chemosensor for selective detection of Fe3+ in aqueous environment with logic gate application. J Fluoresc 26:1021–1028
Ghosh K, Rathi S (2014) A novel probe for selective colorimetric sensing of Fe(ii) and Fe(iii) and specific fluorometric sensing of Fe(iii): DFT calculation and logic gate application. RSC Adv 4:48516–48521
Goswami S, Aich K, Das AK, Manna A, Das S (2013) A naphthalimide–quinoline based probe for selective, fluorescence ratiometric sensing of trivalent ions. RSC Adv 3:2412
Sen S, Sarkar S, Chattopadhyay B, Moirangthem A, Basu A, Dhara K, Chattopadhyay P (2012) A ratiometric fluorescent chemosensor for iron: discrimination of Fe2+ and Fe3+ and living cell application. Analyst 137:3335–3342
Ghosh K, Tarafdar D (2015) Piperazine-based new sensor: selective ratiometric sensing of Fe3+, logic gate construction and cell imaging. Supramol Chem 27:224–232
Akula M, El-Khoury PJ, Nag A, Bhattacharya A (2014) Selective Zn2+ sensing using a modified bipyridine complex. RSC Adv 4:25605
Akula M, Thigulla Y, Nag A, Bhattacharya A (2015) Selective detection of fluoride using fused quinoline systems: effect of pyrrole. RSC Adv 5:57231–57234
Zhang XB, Cheng G, Zhang WJ, Shen GL, Yu RQ (2007) A fluorescent chemical sensor for Fe3+ based on blocking of intramolecular proton transfer of a quinazolinone derivative. Talanta 71:171–177
Wang P, Liu X, Fu J, Chang Y, Yang L, Xu K (2018) Synthesis and fluorescence spectral studies of novel quinolylbenzothiazole-based sensors for selective detection of Fe3+ion. Can J Chem 96:835–841
Xue K, Wang P, Dong W, Luo X, Cheng P, Xu K (2018) Fluorescence Sensors for Fe3+ Ion with High Selectivity and Sensitivity and Bioimaging in Living Cells. Chem Select 3:11081
Wang P, Fu J, Yao K, Chang Y, Xu K, Xu Y (2018) A novel quinoline-derived fluorescent “turn-on” probe for Cu2+ with highly selectivity and sensitivity and its application in cell imaging. Sensors Actuators B Chem 273:1070–1076
Chang Y, Fu J, Yao K, Li B, Xu K, Pang X (2019) Novel fluorescent probes for sequential detection of Cu2+ and citrate anion and application in living cell imaging. Dyes Pigments 161:331–340
Akula M, Padma Sridevi J, Yogeeswari P, Sriram D, Bhattacharya A (2014) New class of antitubercular compounds: synthesis and anti-tubercular activity of 4-substituted pyrrolo[2,3-c]quinolines. Monatsh Chem 145:811–819
Avirah RR, Jyothish K, Ramaiah D (2008) Infrared absorbing croconaine dyes: synthesis and metal ion binding properties. J Org Chem 73:274–279
Ding Y, Xie Y, Li X, Hill JP, Zhang W, Zhu W (2011) Selective and sensitive “turn-on” fluorescent Zn2+ sensors based on di- and tripyrrins with readily modulated emission wavelengths. Chem Commun 47:5431–5433
Akula M, Yogeeswari P, Sriram D, Jha M, Bhattacharya A (2016) Synthesis and anti-tubercular activity of fused thieno-/furo-quinoline compounds. RSC Adv 6:46073–46080
Tang J, Wang L, Mao D, Wang W, Zhang L, Wu S, Xie Y (2011) Ytterbium pentafluorobenzoate as a novel fluorous Lewis acid catalyst in the synthesis of 2,4-disubstituted quinolines. Tetrahedron 67:8465–8469
Damera DP, Venuganti VVK, Nag A (2018) Deciphering the Role of Bilayer of a Niosome towards Controlling the Entrapment and Release of Dyes. Chem Select 3:3930
Acknowledgements
T.U.K. thanks CSIR and BITS-Pilani for PhD fellowship. S.V.P. thanks DST- inspire (IF150605) scheme for providing senior research fellowship. A.N. thanks DST-SERB (SB/FT/CS-129/2013) for financial support. A.B. thanks Council for Scientific and Industrial Research, New Delhi for research grant. Authors gratefully acknowledge support from Department of Science and Technology, India for the FIST grant SR/FST/CSI-240/2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1668 kb)
Rights and permissions
About this article
Cite this article
Kumar, T.U., Pawar, S., Nag, A. et al. Selective Sensing of Iron by Pyrrolo[2,3-c]Quinolines. J Fluoresc 29, 271–277 (2019). https://doi.org/10.1007/s10895-018-02337-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-02337-1