Abstract
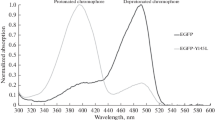
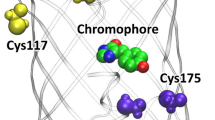
The histidine-modified EGFP was characterized as a sensing element that preferentially binds nanomolar concentrations of Cu2+ in a reversible manner (Kd = 15 nM). This research aims to determine the causes of nanomolar-affinity of this mutant by investigating significant structural and energetic alterations of the chromophore in the presence of different copper ion concentrations. In order to reveal the unknown parts of the quenching mechanism we have elaborated a specific approach that combines theoretical and experimental techniques. The theoretical experiment included the modeling of potential distortions of the chromophores and the corresponding changes in energy using quantum mechanical calculations. Differences between the modeled energy profiles of planar and distorted conformations represented the energies of activation for the chromophore distortions. We found that some values of the experimental activation energies, which were derived from fluorescence lifetime decay analysis (ex: 470 nm, em: 507 nm), were consistent with the theoretical ones. Thus, it has been revealed similarity between the theoretical activation energy (50 kJmol−1) for 40° phenolate-ring distortion and the experimental activation energy (52.17 kJmol−1) required for histidine-modified EGFP saturation with copper. This chromophore conformation was further investigated and it has been found that the large decrease in fluorescence emission is attributed to the significant charge transfer over the molecule which triggers proton transfer thereby neutralizing the cromophore.









Similar content being viewed by others
References
Hsiu-Chuan LV, Chien MT, Tseng YY, Ou KL (2006) Assessment of heavy metal bioavailability in contaminated sediments and soils using green fluorescent protein-based bacterial biosensors. Environ Pollut 142(1):17–23
Hartter DE, Barnea A (1988) Brain tissue accumulates 67copper by two ligand-dependent saturable processes. A high affinity, low capacity and a low affinity, high capacity process. J Biol Chem 263:799–805
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci 158(1):47–52
Desai V, Kaler S (2008) Role of copper in human neurological disorders. Am J Clin Nutr 88(3):855S–858S
Huang GG, Yang J (2003) Selective detection of copper ions in aqueous solution based on an evanescent wave infrared absorption spectroscopic method. Anal Chem 75(10):2262–2269
Lin M, Cho M, Choe WS, Son Y, Lee Y (2009) Electrochemical detection of copper ion using a modified copolythiophene electrode. Electrochim Acta 54(27):7012–7017
Yang M, Yu Y, Shen F, Dierks K, Fang W, Li Q (2010) Detection of copper ion with laser-induced fluorescence in a capillary electrophoresis microchip. Anal Lett 43(18):2883–2891
Isarankura-Na-Ayudhya C, Tantimongcolwat T, Galla HJ, Prachayasittikul V (2010) Fluorescent protein-based optical biosensor for copper Ion quantitation. Biol Trace Elem Res 134(3):352–363
Kneen M, Farinas J, Li Y, Verkman AS (1998) Green fluorescent protein as a noninvasive intracellular pH indicator. Biophys J 74(3):1591–1599
Szabó (P) M, Petres J, Szilágyi L, Miklóssy I, Ábrahám B, Lányi S (2012) Possible application of metal sensitive red fluorescent proteins in environmental monitoring. Environ Eng Manag J 11(1):193–198
Richmond TA, Takahashi TT, Shimkhada R, Bernsdorf J (2000) Engineered metal binding sites on green fluorescence protein. Biochem Biophys Res Commun 268(2):462–465
Palfi M, Kovács E, Miklóssy I, Szilágyi L, Ábrahám B (2009) Engineered green fluorescent protein as a potential metal sensor. Studia Universitatis Babes-Bolyai Chemia, Special Issue: 35–45
Bálint EÉ, Petres J, Szabó M, Orbán CK, Szilágyi L, Ábrahám B (2013) Fluorescence of a histidine-modified enhanced Green Fluorescent Protein (EGFP) effectively quenched by copper(II) ions. J Fluoresc 23(2):273–281
Baker M (2011) Microscopy: bright light, better labels. Nature 478:137–142
Chen MC, Lambert CR, Urgitis JD, Zimmer M (2001) Photoisomerization of green fluorescent protein and the dimensions of the chromophore cavity. Chem Phys 270:157–164
West CW, Hudson AS, Cobb SL, Verlet JRR (2013) Communication: autodetachment versus internal conversion from the S1 state of the isolated GFP chromophore anion. J Chem Phys 139:071104–1–071104–4
Follenius-Wund A, Bourotte M, Schmitt M, Iyice F, Lami H, Bourguignon JJ, Haiech J, Pigault C (2003) Fluorescent derivatives of the GFP chromophore give a new insight into the GFP fluorescence process. Biophys J 85(3):1839–1850
Kummer AD, Wiehler J, Rehaber H, Kompa C, Steipe B, Michel-Beyerle ME (2000) Effects of threonine 203 replacements on excited-state dynamics and fluorescence properties of the Green Fluorescent Protein (GFP). J Phys Chem B 104(19):4791–4798
Jung G, Wiehler J, Zumbusch A (2005) The photophysics of green fluorescent protein: influence of the key amino acids at positions 65, 203, and 222. Biophys J 88(3):1932–1947
Cheng CW, Huang GJ, Hsu HY, Prabhakar C, Lee YP, Diau EWG, Yang JS (2013) Effects of hydrogen bonding on internal conversion of GFP-like chromophores. II. The meta-amino systems. J Phys Chem B 117:2705–2716
Paul BK, Guchhait N (2013) Looking at the Green Fluorescent Protein (GFP) chromophore from a different perspective: a computational insight. Spectrochim Acta A Mol Biomol Spectrosc 103:295–303
Van Den Zegel M, Boens N, Daems D, DeSchryver FC (1986) Possibilities and limitations of the timecorrelated single photon counting technique: a comparative study of correction methods for the wavelength dependence of the instrument response function. Chem Phys 101:311–335
Granovsky AA, Firefly version 8.0.0, http://classic.chem.msu.su/gran/firefly/index.html
Marques MA, López X, Varsano D, Castro A, Rubio A (2003) Time-dependent density-functional approach for biological chromophores: the case of the green fluorescent protein. Phys Rev Lett 90(25):258101-1–258101-4
Fang C, Frontiera RR, Tran R, Mathies RA (2009) Mapping GFP structure evolution during proton transfer with femtosecond Raman spectroscopy. Nature 462:200–204
Lossau H, Kummer A, Heinecke R, Pöllinger-Dammer F, Kompa C, Bieser G, Jonsson T, Silva CM, Yang MM, Youvan DC, Michel-Beyerle ME (1996) Time-resolved spectroscopy of wild-type and mutant green fluorescent proteins reveals excited state deprotonation consistent with fluorophore–protein interactions. Chem Phys 213:1–16
Voityuk AA, Kummer AD, Michel-Beyerle ME, Rösch N (2001) Absorption spectra of the GFP chromophore in solution: comparision of theoretical and experimental results. Chem Phys 269:83–91
Polyakov IV, Grigorenko BL, Epifanovsky EM, Krylov AI, Nemukhin AV (2010) Potential energy landscape of the electronic states of the GFP chromophore in different protonation forms: electronic transition energies and conical intersections. J Chem Theory Comput 6:2377–2387
Philips GN Jr (1996) Structure and dynamics of green fluorescent proteins. Curr Opin Struct Biol 7(6):821–827
Epifanovsky E, Polyakov I, Grigorenko B, Nemukhin A, Krylov AI (2009) Quantum chemical benchmark studies of the electronic properties of the green fluorescent protein chromophore. 1. Electronically excited and ionized states of the anionic chromophore in the gas phase. J Chem Theory Comput 5:1895–1906
Laino T, Nifosí R, Tozzini V (2004) Relationship between structure and optical properties in green fluorescent proteins: a quantum mechanical study of the chromophore environment. Chem Phys 298:17–28
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer Science+Business Media, New York
Hötzer B, Ivanov R, Altmeier S, Kappl R, Jung G (2011) Determination of copper(II) Ion concentration by lifetime measurements of green fluorescent protein. J Fluoresc 21:2143–2153
Huang GJ, Cheng CW, Hsu HY, Prabhakar C, Lee YP, Diau EWG, Yang JS (2013) Effects of hydrogen bonding on internal conversion of GFP-like chromophores. I. The para-amino systems. J Phys Chem B 117:2695–2704
Hasegawa JY, Fujimoto K, Swerts B, Miyahara T, Nakatsuji H (2007) Excited states of GFP chromophore and active site studied by the SAC-CI method: effect of protein-environment and mutations. J Comput Chem 28:2443–2452
Lill MA, Helms V (2001) Proton shuttle in green fluorescent protein studied by dynamic simulations. PNAS 99(5):2778–2781
Arpino JAJ, Rizkallah PJ, Jones DD (2012) Crystal structure of enhanced green fluorescent protein to 1.35 a° resolution reveals alternative conformations for Glu222. PLos One 7(10):e4132/1-8
Sniegowski JA, Phail ME, Wachter RM (2005) Maturation efficiency, trypsin sensitivity, and optical properties of Arg96, Glu222, and Gly67 variants of green fluorescent protein. Biochem Biophys Res Commun 332:657–663
Voityuk AA, Michel-Beyerle ME, Rösch N (1998) Quantum chemical modeling of structure and absorption spectra of the chromophore in green fluorescent proteins. Chem Phys 231:13–25
Acknowledgments
The work has been funded by the Sectorial Operational Programme Human Resources Development 2007–2013 of the Romanian Ministry of Labour, Family and Social Protection through the Financial Agreement POSDRU/88/1.5/S/60203 and by the University of Sapientia, Department of Bioengineering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Péterffy, J.(., Szabó, M., Szilágyi, L. et al. Fluorescence of a Histidine-Modified Enhanced Green Fluorescent Protein (EGFP) Effectively Quenched by Copper(II) Ions. Part II. Molecular Determinants. J Fluoresc 25, 871–883 (2015). https://doi.org/10.1007/s10895-015-1567-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1567-4