Abstract
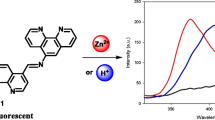
A novel blue-emitting 1,8-naphthalimide fluorophore designed as a molecular PET-based probe for determination of pH and detection of transition metal ions in the environment was successfully synthesized. Novel compound was configured on the “fluorophore-spacer-receptor” format. Due to the tertiary amine receptor the novel system showed “off-on” switching properties under the transition from alkaline to acid media (FE = 3.2) and in the presence of Zn2+ ions (FE = 2.5). The results obtained illustrate the high potential of the synthesized blue-emitting 1,8-naphthalimide fluorophore as an efficient pH chemosensing material and a selective probe for Zn2+ ions.

ᅟ










Similar content being viewed by others
Abbreviations
- PET:
-
Photoinduced electron transfer
- ICT:
-
Internal charge transfer
- FE:
-
Fluorescence enhancement
References
Sareen D, Kaur P, Singh K (2014) Strategies in detection of metal ions using dyes. Coord Chem Rev 265:125–154
Bojinov V, Georgiev N (2011) Molecular sensor and molecular logic gates. J Univ Chem Technol Met (Sofia) 46:3–26
Georgiev N, Bryaskova R, Tzoneva R, Ugrinova I, Detrembleur C, Miloshev S, Asiri A, Qusti A, Bojinov V (2013) A novel pH sensitive water soluble fluorescent nanomicellar sensor for potential biomedical applications. Bioorg Med Chem 21:6292–6302
Li X, Gao X, Shi W, Ma H (2014) Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem Rev 114:590–659
Brown G, de Silva A, James M, McKinney B, Pears D, Weir S (2008) Solid-bound, proton-driven, fluorescent “off-on-off” switches based on PET (photoinduced electron transfer). Tetrahedron 64:8301–8306
Bojinov V, Konstantinova T (2007) Fluorescent 4-(2,2,6,6-tetramethylpiperidin-4-ylamino)-1,8-naphthalimide pH chemosensor based on photoinduced electron transfer. Sens Actuators B: Chem 123:869–876
Bojinov V, Georgiev N, Nikolov P (2008) Design and synthesis of core and peripherally functionalized with 1,8-naphthalimide units fluorescent PAMAM dendron as light harvesting antenna. J Photochem Photobiol A Chem 197:281–289
de Silva A, Uchiyama S (2011) Molecular logic gates and luminescent sensors based on photoinduced electron transfer. Top Curr Chem 300:1–28
Bojinov V, Panova I (2009) Novel 4-(2,2,6,6-tetramethylpiperidin-4-ylamino)-1,8-naphthalimide based yellow-green emitting fluorescence sensors for transition metal ions and protons. Dyes Pigments 80:61–66
Bojinov V, Georgiev N, Bosch P (2009) Design and synthesis of highly photostable yellow-green emitting 1,8-naphthalimides as fluorescent sensors for metal cations and protons. J Fluoresc 19:127–139
Georgiev N, Dimov S, Asiri A, Alamry K, Obaid A, Bojinov V (2014) Synthesis, selective pH-sensing activity and logic behavior of highly water-soluble 1,8-naphthalimide and dihydroimidazonaphthalimide derivatives. J Lumin 149:325–332
Ramachandram B, Saroja G, Sankaran N, Samanta A (2000) Unusually high fluorescence enhancement of some 1,8-naphthalimide derivatives induced by transition metal salts. J Phys Chem B 104:11824–11832
Bojinov V, Panova I, Chovelon J-M (2008) Novel blue emitting tetra- and pentamethylpiperidin-4-yloxy-1,8-naphthalimides as photoinduced electron transfer based sensors for transition metal ions and protons. Sens Actuators B: Chem 135:172–80
Falchuk K (1998) The molecular basis for the role of zinc in developmental biology. Mol Cell Biochem 188:41–48
Frederickson C, Koh J, Bush A (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Bush A, Pettingell W, Multhaup G, Paradis M, Vonsattel J-P, Gusella J, Beyreuther K, Masters C, Tanzi R (1994) Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265:1464–1467
Walker C, Black R (2004) Zinc and the risk for infectious disease. Annu Rev Nutr 24:255–75
Choi D, Koh J (1998) Zinc and brain injury. Annu Rev Neurosci 21:347–375
O’Halloran T (1993) Transition metals in control of gene expression. Science 261:715–725
Jin W, Jiang J, Wang X, Zhu X, Wang G, Song Y, Ba C (2011) Continuous intra-arterial blood pH monitoring in rabbits with acid–base disorders. Respir Physiol Neurobiol 177:183–188
Han J, Burgess K (2010) Fluorescent indicators for intracellular pH. Chem Rev 110:2709–2728
Li C, Zhou Y, Xu F, Li Y, Zou C, Weng C (2012) A fluorescent pH chemosensor based on functionalized naphthalimide in aqueous solution. Anal Sci 28:743–747
Bojinov V, Panova I, Simeonov D, Georgiev N (2010) Synthesis and sensor activity of photostable blue emitting 1,8-naphthalimides containing s-triazine UV absorber and HALS fragments. J Photochem Photobiol A Chem 210:89–99
Panah H, Khosravi A, Gharanjig K (2010) Synthesis and characterization of new fluorescent polymerizable dyes based on naphthalimide. Iran Polym J 19:491–500
Bojinov V, Simeonov D (2010) Synthesis of highly photostable blue emitting 1,8-naphthalimides and their acrylonitrile copolymers. Polym Degrad Stab 95:43–52
Martin E, Weigand R, Pardo A (1996) Solvent dependence of the inhibition of intramolecular charge-transfer in N-substituted 1,8-naphthalimide derivatives as dye lasers. J Lumin 68:157–164
Zhang Y, Zhou C (2011) Synthesis and activities of naphthalimide azoles as a new type of antibacterial and antifungal agents. Bioorg Med Chem Lett 21:4349–4352
Ott I, Xu Y, Qian X (2011) Fluorescence properties and antiproliferative effects of mono-, bis-, and tris- thiophenylnaphthalimides: Results of a comparative pilot study. J Photochem Photobiol B Biol 105:75–80
de Souza M, Correa R, Filho V, Grabchev I, Bojinov V (2002) 4-Nitro-1,8-naphthalimides exhibit antinociceptive properties. Pharmazie 56:430–431
Georgiev N, Asiri A, Qusti A, Alamry K, Bojinov V (2014) A pH sensitive and selective ratiometric PAMAM wavelength-shifting bichromophoric system based on PET, FRET and ICT. Dyes Pigments 102:35–45
Bojinov V, Simeonov D, Georgiev N (2008) A novel blue fluorescent 4-(1,2,2,6,6-pentamethylpiperidin-4-yloxy)-1,8-naphthalimide pH chemosensor based on photoinduced electron transfer. Dyes Pigments 76:41–46
Jiang J, Leng B, Xiao X, Zhao P, Tian H (2009) “Off-On-Off” fluorescent proton switch synthesized by RAFT polymerization. Polymer 50:5681–5684
Georgiev N, Asiri A, Alamry K, Obaid A, Bojinov V (2014) Selective ratiometric pH-sensing PAMAM light-harvesting dendrimer based on Rhodamine 6G and 1,8-naphthalimide. J Photochem Photobiol A Chem 277:62–74
Gan J, Song Q, Hou X, Chen K, Tian H (2004) 1,8-Naphthalimides for non-doping OLEDs: the tunable emission color from blue, green to red. J Photochem Photobiol A Chem 162:399–406
Georgiev N, Bojinov V, Venkova A (2013) Design, synthesis and pH sensing properties of novel PAMAM light-harvesting dendrons based on rhodamine 6G and 1,8-naphthalimide. J Fluoresc 23:459–471
Marinova N, Bojinov V, Georgiev N (2011) Design, synthesis and pH sensing properties of novel 1,8-naphtalimide-based bichromophoric system. J Photochem Photobiol A Chem 222:132–140
Georgiev N, Bojinov V, Marinova N (2010) Novel PAMAM light-harvesting antennae based on 1,8-naphthalimide: Synthesis, energy transfer, photophysical and pH sensing properties. Sens Actuators B: Chem 150:655–666
Georgiev N, Bojinov V (2010) The design and synthesis of a novel 1,8-naphthalimide PAMAM light-harvesting dendron with fluorescence “off-on” switching core. Dyes Pigments 84:249–256
Wang Y, Zhang X, Han B, Peng J (2010) The synthesis and photoluminescence characteristics of novel blue light-emitting naphthalimide derivatives. Dyes Pigments 86:190–196
Grabchev I, Moneva I, Bojinov V, Guittonneau S (2000) Synthesis and properties of fluorescent 1,8-naphthalimide dyes for application in liquid crystal displays. J Mater Chem 10:1291–1296
Ferreira R, Remon P, Pischel U (2009) Multivalued logic with a tristable fluorescent switch. J Phys Chem C 113:5805–5811
Marinova N, Georgiev N, Bojinov V (2013) Facile synthesis, sensor activity and logic behaviour of 4-aryloxy substituted 1,8-naphthalimide. J Photochem Photobiol A Chem 254:54–61
Georgiev N, Lyulev M, Bojinov V (2012) Sensor activity and logic behavior of PET based dihydroimidazonaphthalimide diester. Spectrochim Acta Part A 97:512–520
Georgiev N, Yaneva I, Surleva A, Asiri A, Bojinov V (2013) Synthesis, sensor activity and logic behavior of a highly water-soluble naphthalimide derivative. Sens Actuators B: Chem 184:54–63
Yang L, Yang W, Xu D, Zhang Z, Liu A (2013) A highly selective and sensitive Fe3+ fluorescent sensor by assembling three 1,8-naphthalimide fluorophores with a tris(aminoethylamine) ligand. Dyes Pigments 97:168–174
Liu B, Tian H (2005) A ratiometric fluorescent chemosensor for fluoride ions based on a proton transfer signaling mechanism. J Mater Chem 15:2681–2686
Brouwer A (2011) Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl Chem 83:2213–2228
Georgiev N, Bojinov V, Nikolov P (2009) Design and synthesis of a novel pH sensitive core and peripherally 1,8-naphthalimide-labeled PAMAM dendron as light harvesting antenna. Dyes Pigments 81:18–26
Bojinov V, Panova I (2008) Photo-stability of yellow-green emitting 1,8-naphthalimides containing built-in s-triazine UV absorber and HALS fragments and their acrylonitrile copolymers. Polym Degrad Stab 93:1142–1150
Bojinov V, Panova I, Simeonov D (2008) Design and synthesis of polymerizable, yellow-green emitting 1,8-naphthalimides containing built-in s-triazine UV absorber and hindered amine light stabilizer fragments. Dyes Pigments 78:101–110
Georgiev N, Bojinov V (2011) Design, synthesis and photostability of novel 1,8-naphthalimide PAMAM Light-harvesting dendrons. J Fluoresc 21:51–63
Georgiev N, Asiri A, Qusti A, Alamry K, Bojinov V (2014) Design and synthesis of pH-selective fluorescence sensing PAMAM light-harvesting dendrons based on 1,8-naphthalimides. Sens Actuators B: Chem 190:185–198
Bojinov V, Georgiev N, Marinova N (2010) Design and synthesis of highly photostable fluorescence sensing 1,8-naphthalimide-based dyes containing s-triazine UV absorber and HALS units. Sens Actuators B: Chem 148:6–16
Liu J, de Silva A (2012) Path-selective photoinduced electron transfer (PET) in a membrane-associated system studied by pH-dependent fluorescence. Inorg Chim Acta 381:243–246
Georgiev N, Bojinov V (2012) Design, synthesis and sensor activity of a highly photostable blue emitting 1,8-naphthalimide. J Lumin 132:2235–2241
Ramachandram B (2005) Fluorescence sensor design for transition metal ions: The role of the PIET interaction efficiency. J Fluoresc 15:71–83
Bojinov V, Georgiev N, Nikolov P (2008) Synthesis and photophysical properties of fluorescence sensing ester- and amidoamine-functionalized 1,8-naphthalimides. J Photochem Photobiol A Chem 193:129–138
Georgiev N, Bojinov V, Nikolov P (2011) The design, synthesis and photophysical properties of two novel 1,8-naphthalimide fluorescent pH sensors based on PET and ICT. Dyes Pigments 88:350–357
Ramachandram B, Sankaran N, Karmakar R, Saha S, Samanta A (2000) Fluorescence signalling of transition metal ions by multi-component systems comprising 4-chloro-1,8-naphthalimide as fluorophore. Tetrahedron 56:7041–7044
Soni M, Das S, Sahu P, Kar U, Rahaman A, Sarkar M (2013) Synthesis, photophysics, live cell imaging, and aggregation behavior of some structurally similar alkyl chain containing bromonaphthalimide systems: Influence of alkyl chain length on the aggregation behavior. J Phys Chem C 117:14338–14347
Kasha M, Rawls H, El-Bayoumi M (1965) The exciton model in molecular spectroscopy. Pure Appl Chem 11:371–392
Attia M, Youssef A, El-Sherif R (2014) Durable diagnosis of seminal vesicle and sexual gland diseases using the nano optical sensor thin film Sm-doxycycline complex. Anal Chim Acta 835:56–64
Acknowledgments
This work was supported by the National Science Foundation of Bulgaria (project DDVU-02/97). Authors also acknowledge the Science Foundation at the University of Chemical Technology and Metallurgy (Sofia, Bulgaria).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimov, S.M., Georgiev, N.I., Asiri, A.M. et al. Synthesis and Sensor Activity of a PET-based 1,8-naphthalimide Probe for Zn2+ and pH Determination. J Fluoresc 24, 1621–1628 (2014). https://doi.org/10.1007/s10895-014-1448-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1448-2