Abstract
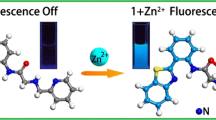
The synthesis and properties of a new compound, viz., (N,N′-[1,10-phenanthroline-4,7-diyldi((E)methylylidene)]bis(1,10-phenanthrolin-5-amine) (1), is described. Compound 1 can be used as a selective fluorescent Zn2+ sensor in buffered solution. Furthermore, 1 induces turn on fluorogenic response to H+ ions. Finally, it is shown that an OR logic gate can be constructed with 1 by using Zn2+ and H+ as two-inputs.

In this paper, the design, synthesis and physicochemical properties of a new compound 1 based on 1,10-phenanthroline scaffold, is reported. It is noted that 1 can be used as a selective fluorescent Zn2+ sensor in 0.01 M HEPES buffer containing DMF (2 % v/v, pH = 7.4) at room temperature. Furthermore, the spectrophotometric results suggest that compound 1 can be used as a pH reporter in highly acidic conditions (pH < 5). Finally, it was also shown that an OR logic gate can be constructed with 1 by using Zn2+ and H+ as two-inputs.








Similar content being viewed by others
References
Czarnik AW (1993) Fluorescent chemosensors for ion and Molecule recognition. American Chemical Society, Washington DC
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Bush AI (2003) The metallobiology of Alzheimer’s disease. Trends Neurosci 26:207–214
Chausmer AB (1998) Zinc, insulin and diabetes. J Am Coll Nutr 17:109–115
de Silva AP, Fox DB, Huxley AJM (2000) Combining luminescence, coordination and electron transfer for signalling purposes. Coord Chem Rev 205:41–57
Qu L, Yin C, Huo F, Chao J, Zhang Y, Cheng F (2014) A pyridoxal-based dual chemosensor for visual detection of copper ion and ratiometric fluorescent detection of zinc ion. Sens Actuators, B 191:158–164
Ma Y, Chen H, Wang F, Kambam S, Wang Y, Mao C, Chen X (2014) A highly sensitive and selective ratiometric fluorescent sensor for Zn2+ ion based on ICT and FRET. Dyes Pigments 102:301–307
Wei TB, Zhang P, Shi BB, Chen P, Lin Q, Liu J, Zhang YM (2013) A highly selective chemosensor for colorimetric detection of Fe3+ and fluorescence turn-on response of Zn2+. Dyes Pigments 97:297–302
Lin W, Buccella D, Lippard SJ (2013) Visualization of Peroxynitrite-Induced changes of Labile Zn2+ in the Endoplasmic Reticulum with Benzoresorufin-Based fluorescent probes. J Am Chem Soc 135:13512–13520
Sarkar D, Pramanik AK, Mondal TK (2014) Coumarin based fluorescent ‘turn-on’ chemosensor for Zn2+: an experimental and theoretical study. J Lumin 146:480–485
Tsay OG, Manjare ST, Kim H, Lee KM, Lee YS, Churchill DG (2013) Novel Reversible Zn2+-Assisted Biological Phosphate “Turn-On” Probing through Stable Aryl-hydrazone Salicylaldimine Conjugation That Attenuates Ligand Hydrolysis. Inorg Chem 52:10052–10061
Li K, Tong A (2013) A new fluorescent chemosensor for Zn2+ with facile synthesis: “turn-on” response in water at neutral pH and its application for live cell imaging. Sens Actuators, B 184:248–253
Helal A, Rashid MHO, Choi CH, Kim HS (2012) New regioisomeric naphthol-substituted thiazole based ratiometric fluorescence sensor for Zn2+ with a remarkable red shift in emission spectra. Tetrahedron 68:647–653
You QH, Chan PS, Chan WH, Hau SCK, Lee AWM, Mak NK, Mak TCW, Wong RNS (2012) A quinolinyl antipyrine based fluorescence sensor for Zn2+ and its application in bioimaging. RSC Adv:11078–11083
Zhang B, Cao KS, Xu ZA, Yang ZQ, Chen HW, Huang W, Yin G, You XZ (2012) Cell-compatible fluorescent chemosensor for Zn2+ based on a 3,8-extended 1,10-phenanthroline derivative. Eur J Inorg Chem:3844–3851
Yin S, Zhang J, Feng H, Zhao Z, Xu L, Qiu H, Tang B (2012) Zn2+-selective fluorescent turn-on chemosensor based on terpyridine-substituted siloles. Dyes Pigments 95:174–179
Li YP, Yang HR, Zhao Q, Song WC, Han J, Bu XH (2012) Ratiometric and selective fluorescent sensor for Zn2+ as an “off–on–off” Switch and logic gate. Inorg Chem 51:9642–9648
Zhou Y, Li ZX, Zang SQ, Zhu YY, Zhang HY, Hou HW, Mak TCW (2012) A novel sensitive turn-on fluorescent Zn2+ chemosensor based on an Easy to Prepare C 3-Symmetric Schiff-Base derivative in 100 % aqueous solution. Org Lett 14:1214–1217
Fu Y, Xing Z, Zhu C, Yang H, He W, Zhu C, Cheng Y (2012) A novel calixsalen macrocycle: metal sensing behavior for Zn2+ and intracellular imaging application. Tetrahedron Lett 53:804–807
Gao C, Jin X, Yan X, An P, Zhang Y, Liu L, Tian H, Liu W, Yao X, Tang Y (2013) A small molecular fluorescent sensor for highly selectivity of zinc ion. Sens Actuators B 176:775–781
Choi JY, Kim D, Yoon J (2013) A highly selective “turn-on” fluorescent chemosensor based on hydroxy pyrene–hydrazone derivative for Zn2+. Dyes Pigments 96:176–179
Kim MJ, Kaur K, Singh N, Jang DO (2012) Benzimidazole-based receptor for Zn2+ recognition in a biological system: a chemosensor operated by retarding the excited state proton transfer. Tetrahedron 68:5429–5433
Coskun A, Deniz E, Akkaya EU (2007) A sensitive fluorescent chemosensor for anions based on a styryl-boradiazaindacene framework. Tetrahedron Lett 48:5359–5361
Aka FN, Akkaya MS, Akkaya EU (2001) Remarkable cooperative action of two zinc centers in the hydrolysis of plasmid DNA. J Mol Catal A-Chem 165:291–294
Atilgan S, Ozdemir T, Akkaya EU (2008) A sensitive and selective ratiometric near IR fluorescent probe for zinc ions based on the distyryl-bodipy fluorophore. Org Lett 10:4065–4067
Accorsi G, Listorti A, Yoosaf K, Armaroli N (2009) 1,10-Phenanthrolines: versatile building blocks for luminescent molecules, materials and metal complexes. Chem Soc Rev 38:1690–1700
Yang C, Xu J, Chen W, Lu M, Li Y, Wang XJ (2014) A novel colorimetric and fluorescent sensor for fluoride detection based on a three-arm phenanthroline derivative. J Mater Sci 4:7040–7048
Pamuk M, Algi F (2012) Synthesis of a novel on/off fluorescent cadmium(II) probe. Tetrahedron Lett 53:7010–7012
Karakaya S, Algi F (2014) A novel dual channel responsive zinc(II) probe. Tetrahedron Lett 55:5555–5559
Yoldas A, Algi F (2015) An imidazo-phenanthroline scaffold enables both chromogenic Fe(II) and fluorogenic Zn(II) detection. RSC Adv 5:7868–7873
Algi MP (2016) A fluorescent hypochlorite probe Built on 1,10-phenanthroline scaffold and its ion recognition Features. J Fluoresc 26:487–496
Algi MP (2016) A highly selective dual channel hypochlorite probe based on fluorescein and 1,10-phenanthroline. Tetrahedron 72:1558–1565
de Silva AP, Dixon IM, Gunaratne HQN, Gunnlaugsson T, Maxwell PRS, Rice TE (1999) Integration of logic Functions and Sequential Operation of Gates at the Molecular-Scale. J Am Chem Soc 121:1393–1394
Feringa B (2001) For molecular logic systems, see: Molecular Switches; Ed.; Wiley-VCH: Weinheim, Germany, pp. 339–361.
de Silva AP, Uchiyama S (2007) Molecular logic and computing. Nat. Nanotech 2:399–410
Coskun A, Baytekin BT, Akkaya EU (2003) Novel fluorescent chemosensor for anions via modulation of oxidative PET: a remarkable 25-fold enhancement of emission. Tetrahedron Lett 44:5649–5651
Turfan B, Akkaya EU (2002) Modulation of boradiazaindacene emission by cation-mediated oxidative PET. Org Lett 4:2857–2859
Liu T, Hu J, Yin J, Zhang Y, Li C, Liu S (2009) Enhancing detection sensitivity of responsive microgel-based Cu(II) chemosensors via thermo-induced volume phase transitions. Chem Mater 21:3439–3446
de Silva AP, Gunaratne HQN, Gunnlaugsson T, McCoy CP, Maxwell PRS, Rademacher JT, Rice TE (1996) Photoionic devices with receptor-functionalized fluorophores. Pure Appl Chem 68:1443–1448
de Silva AP, Moody TS, Wright GD (2009) Fluorescent PET (Photoinduced electron transfer) sensors as potent analytical tools. Analyst 134:2385–2393
de Silva AP (2012) Mid-infrared frequency combs. Nat Chem 4:440–449
Valeur B (2002) Molecular fluorescence: Principles and Applications. Wiley-VCH, Weinheim, Germany, p. 90
Algi MP, Oztas Z, Algi F (2012) Triple channel responsive Cu2+ probe. Chem Commun 48:10219–10221
Oztas Z, Pamuk M, Algi F (2013) Nonreaction-based fluorescent Au3+ probe that gives fast response in aqueous solution. Tetrahedron 69:2048–2051
Acknowledgments
M.P.A is indebted to Aksaray University for partial financial support of this work (ASU BAP 2015-092).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1622 kb)
Rights and permissions
About this article
Cite this article
Algi, M.P. A Simple and Selective Fluorescent Sensor for Zn2+ and H+ Ions in Aqueous Solution with OR Logic Gate Function. J Fluoresc 26, 1083–1089 (2016). https://doi.org/10.1007/s10895-016-1798-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1798-z