Abstract
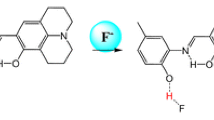
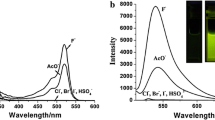
A simple colorimetric and ratiometric fluorescent anion sensor 1, was rationally designed and synthesized by facile one-step condensation on basis of the mechanism of intramolecular charge transfer (ICT). The sensor 1 shows more highly and selectively ability to F− that induced the color changes from little yellow to purple, absorption-transferred to long wavelength and emission-transferred to short wavelength. Accordingly, 1H NMR analysis reveals that the F−-induced colorimetric and fluorometric responses of 1 are simply driven by hydrogen bonding interaction between the NH protons and F− ions.









Similar content being viewed by others
References
Beer PD, Gale PA (2001) Angew Chem 113:502–32
Beer PD, Gale PA (2001) Angew Chem Int Ed 40:486–516
Martínez-Mañez R, Sancenón F (2003) Chem Rev 103:4419–76
Kubik S, Reyheller C, Stüwe S (2005) J Incl Phenom Macrocycl Chem 52:137–87
Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM (2006) Coord Chem Rev 250:3094–117
Shao J, Yu M, Lin H, Lin HK (2008) Spectrochim Acta A Mol Biomol Spectrosc 70:1217–1221
Ayoob S, Gupta AK (2006) Crit Rev Environ Sci Technol 36:433–487
Liu B, Tian H (2005) J Mater Chem 15:2681–2686
Gunnlaugsson T, Davis AP, O’Brien JE, Glynn M (2002) Org Lett 4:2449–2452
Grases F, March JG (1990) Anal Chim Acta 229:249–254
Baird C, Cann M (2005) Environmental chemistry. Freeman, New York
Pu L (2004) Chem Rev 104:1687–1716
de Silva AP, Gunarame HQN, Habib-Jiwan J, McCoy CP, Rice TE, Soumillion J (1995) Angew Chem Int Ed 34:1728–1731
Roland K (1998) Angew Chem Int Ed 37:772–773
Gunnlaugsson T, Leonard JP, Murray NS (2004) Org Lett 6:1557–1560
Bondy CR, Loeb SJ (2003) Coord Chem Rev 240:77–99
Chmielewski MJ, Jurczak J (2005) Chem Eur J 11:6094
Sessler JL, Camiolo S, Gale PA (2003) Coord Chem Rev 240:17–55
Kang SO, Llinares JM, Powell D, VanderVelde D, Bowman-James K (2003) J Am Chem Soc 125:10152–10153
Beer PD, Stokes SE (1995) Polyhedron 14:873–879
Brooks SJ, Gale PA, Light ME (2006) Chem Commun 4344–4346
Gale PA (2005) Chem Commun 3761–3772
Pfeffer FM, Gunnlaugsson T, Jensen P, Kruger PE (2005) Org Lett 7:5357
Suk J-M, Chae MK, Kim N-K, Kim U-L, Jeong K-S (2008) Pure Appl Chem 80:599–608
Zielínski T, Dydio P, Jurczak J (2007) Tetrahedron 64:568–574
Chmielewski MJ, Zhao L, Brown A, Curiel D, Sambrook MR, Thompson AL, Santos SM, Felix V, Davis JJ, Beer PD (2008) Chem Commun 3154–3156
Piatek P, Lynch VM, Sessler JL (2004) J Am Chem Soc 126:16073–16076
Pfeffer FM, Lim KF, Sedgwick KJ (2007) Org Biomol Chem 5:1795–1799
Gale PA (2008) Chem Commun 4525–4540
Peng X, Wu Y, Fan J, Tian M, Han KJ (2005) Org Chem 70:10524–10531
Zhang Y, Guo X, Si W, Jia L, Qian X (2008) Org Lett 10:473–476
Yang H, Yi T, Zhou Z, Zhou Y, Wu J, Xu M, Li F, Huang C (2007) Langmuir 23:8224–8230
Shao J, Lin H, Lin HK (2008) Talanta 75:1015–1020
Shao J, Lin H, Yu M, Lin HK (2008) Talanta 75:551–555
Shao J, Lin H, Shang XF, Chen HM, Lin HK (2007) J Incl Phenom Macrocycl Chem 59:371–375
Xu Z, Xiao Y, Qian X, Cui J, Cui D (2005) Org Lett 889–892
Valeur B (2001) Molecular fluorescence: principles and applications. Weinheim, NewYork, pp 298–299
Shao J, Lin H, Lin HK (2008) Talanta 77:273–277
Connors KA (1987) Binding constants: the measurement of molecular complex stability. Wiley, New York, pp 25–28
Bourson J, Pouget J, Valeur B (1993) J Phys Chem 97:4552–4556
Korendovych IV, Cho M, Butler PL, Staples RJ, Rybak-Akimova EV (2006) Org Lett 8:3171–3174
Palacios MA, Nishiyabu R, Marquez M, Anzenbacher P Jr (2007) J Am Chem Soc 129:7538–7544
Song F, Garner AL, Koide K (2007) J Am Chem Soc 129:12354–12355
Acknowledgments
This project was supported by the National Natural Science Foundation of China (20371028, 20671052).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Lin, H. & Lin, H. Ratiometric and Selective Fluorescent Sensor for F− Based on Intramolecular Charge Transfer (ICT). J Fluoresc 20, 1299–1305 (2010). https://doi.org/10.1007/s10895-010-0681-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0681-6