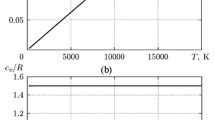
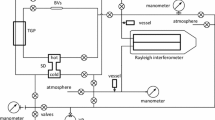
The area of application of the rarefied neutral methane–nitrogen gas mixture is considered. Experimental data on the transport properties of this mixture and its components were analyzed and generalized on the basis of molecular-kinetic theory relations with the use of the potentials of pair uniform and cross interactions of CH4 and N2 molecules. The parameters of three spherical symmetric three-parameter m-6 Lennard-Jones interaction potentials with a repulsive branch of varying rigidity were determined with the use of the nonlinear weight method of least squares. Tables of reference data on the viscosity of the indicated mixture and the coefficients of interdiffusion of its components were calculated for the concentration range 0–1 at temperatures 100–1150 K. Estimates of the confidential errors in determining the properties of this mixture have been made with the use of the error matrix of parameters of the indicated potentials. The results of calculations were compared with the corresponding reference data obtained earlier for the CH4–N2 gas mixture.
Similar content being viewed by others
References
A. Boushehri, J. Bzowski, J. Kestin, and E. A. Mason, Equilibrium and transport properties of eleven polyatomic gases at low densities, J. Phys. Chem. Ref. Data, 16, No. 3, 445–466 (1987).
J. Bzowski, J. Kestin, E. A. Mason, and F. J. Uribe, Equilibrium and transport properties of gas mixtures at low densities: Eleven polyatomic gases and five noble gases, J. Phys. Chem. Ref. Data, 19, No. 5, 1176–1231 (1990).
A. N. Kalashnikov, A System of Reference Data on Kinetic Coefficients for Calculating the Processes of Transfer in the Gas–Air System of a Boiler Plant, Candidate′s Dissertation (in Engineering), OIVT RAN, Moscow (2001).
L. R. Fokin and A. N. Kalashnikov, Viscosity and self-diffusion coefficient of a rarefied steam. Refinement of reference data, Teplofiz. Vys. Temp., 46, No. 5, 674–679 (2008).
L. R. Fokin and A. N. Kalashnikov, Transport properties of mixtures of rarefied N2–H2 gases in the EPIDIF data base, Teplofiz. Vys. Temp., 47, No. 5, 675–687 (2009).
A. G. Shashkov, A. F. Zolotukhina, L. R. Fokin, and A. N. Kalashnikov, Transport properties of mixtures of rarefied neutral gases. Nitrogen–argon system, J. Eng. Phys. Thermophys., 83, No. 1, 188–208 (2010).
L. R. Fokin, A. N. Kalashnikov, and A. F. Zolotukhina, Transport properties of mixtures of rarefied gases. Nitrogen–methane system, J. Eng. Phys. Thermophys., 84, No. 6, 1408–1420 (2011).
V. N. Popov, L. R. Fokin, and A. N. Kalashnikov, Analytical representation of collision integrals for the m-6 L-J potential, Teplofiz. Vys. Temp., 37, No. 1, 49–55 (1999).
L. Zarkova, U. Hohm, and M. Damyanova, Viscosity and pVT-second virial coefficient of binary noble-globular gas and globular-globular gas mixtures calculated by means of an isotropic temperature-dependent potential, J. Phys. Chem. Ref. Data, 32, No. 4, 1591–1605 (2003).
S. Palle and R. S. Miller, Analysis of high-pressure hydrogen, methane, and heptane laminar diffusion flames: Thermal diffusion factor modeling, Combust. Flame, 151, No. 4, 581–600 (2007).
K. C. Clay, S. P. Speakman, G. A. J. Amaratunga, and S. R. P. Silva, Characterization of a-C:H:N deposition from CH4/N2 rf plasmas using optical emission spectroscopy, J. Appl. Phys., 79, No. 6, 7229–7233 (1996).
A. Luspay-Kuti, V. F. Chevrier, F. C. Wasiak, et al., Experimental simulations of CH4 evaporation on Titan, Geophys. Res. Lett., 39, L23203 (5) (2012).
C. R. Mueller and R. W. Cahil, Mass spectrometric measurement of diffusion coefficient, J. Chem. Phys., 40, No. 3, 651–654 (1964).
I. F. Golubev, Viscosity of Gases and Gas Mixtures [in Russian], Fizmatgiz, Moscow (1959).
N. E. Gnezdilov and I. F. Golubev, Viscosity of the methane–nitrogen and methane–nitrogen–hydrogen mixtures at temperatures from 273 to 473 K and pressures of up to 490.3·105 N/m2, Gazov. Prom., No. 4, 46–48 (1968).
A. E. Humphreys and R. Gray, Thermal diffusion as a probe of binary diffusion coefficient at elevated temperatures. II. CH4–N2 and CH4–CO2, Proc. Roy. Soc. (London) A, 322, No. 1548, 89–100 (1971).
J. Engel and H. Knaap, Experimental determination of binary diffusion coefficients in gaseous systems He–CH4, He–N2, CH4–N2, Wärme- und Stoffübertragung, 6, No. 3, 146–152 (1973).
W. A. Wakeham and D. H. Slater, Diffusion coefficients for n-alkanes in binary gaseous mixtures with nitrogen, J. Phys. B, 6, 886–896 (1973).
A. K. Pal, S. K. Bhattacharyya, and A. K. Barua, Thermal diffusion in polyatomic gas mixtures CH4–N2 and CH4–CO2, J. Phys. B, 7, No. 1, 178–184 (1974).
J. Kestin and S. T. Ro, The viscosity of nine binary and two ternary mixtures of low density, Ber. Bunsen Ges. Phys. Chem., 78, No. 1, 20–23 (1974).
T. El Hawary, Messung der Dichte und Viskosität in Gasphase von Methan, Stickstoffund Methan–Stickstoff–Gemischen, Dissertation, Ruhr-Universität Bochum (2009).
A. F. Bogatyrev, O. D. Makeenkova, and M. A. Nezovitinova, Experimental study of thermal diffusion in multicomponent gaseous mixtures, Int. J. Thermophys., 36, No. 4, 633–647 (2015).
A. G. Shashkov, A. F. Zolotukhina, and V. B. Vasilenko, The Factor of Thermal Diffusion of Gas Mixtures. Determination Methods [in Russian], Belorusskaya Nauka, Minsk (2007).
S. A. Losev (Ed.), Models of the Processes of Molecular Transfer in the Physicochemical Gas Dynamics: Physicochemical Processes in Gas Dynamics [in Russian], Vol. 3, Fizmatlit, Moscow (2012).
E. A. Maison, Transfer in a neutral gas, in: Kinetic Processes in Gases and Plasmas [Russian translation], Atomizdat, Moscow (1972), pp. 52–91.
V. B. Leonas, Short-range intermolecular forces, Usp. Fiz. Nauk, 107, Issue 1, 29–55 (1972).
H. Schindler, R. Vogelsang, V. Staemmler, et al., Ab initio intermolecular potentials of methane, nitrogen and methane + nitrogen and their use in Monte Carlo simulations of fluids and fluid mixtures, Mol. Phys., 88, No. 6, 1413–1429 (1993).
M. Shadman, S. Veganegi, and F. Ziaie, Ab initio interaction potential of methane and nitrogen, Chem. Phys. Lett., 467, 237–242 (2009).
Yu. N. Kalugina, V. N. Cherepanov, M. A. Buldakov, et al., Theoretical investigation of the potential energy surface of the Van der Waals complex CH4–N2, J. Chem. Phys., 131, 134304 (8) (2009).
D. R. Lide (Ed.), CRC Handbook of Chemistry and Physics, 7th edn., CRC Press, Boca Raton (1993), Ch. 6, pp. 205–206.
T. S. Storvik and E. A. Mason, Determination of diffusion coefficient from viscosity measurements: effect of higher Chapman–Enskog approximations, J. Chem. Phys., 45, No. 10, 3752–3754 (1966).
A. Maghari and A. H. Jaili, Calculation of transport coefficients for CH4–N2 and CH4–O2 by the inversion method, J. Phys. Soc. Jpn., 73, No. 5, 1191–1196 (2004).
J. Moghadasi, M. M. Papari, and F. Yousefi , Transport coefficients of natural gases, J. Chem. Eng. Jpn., 40, No. 9, 698–710 (2007).
I. G. Kaplan, Introduction to the Theory of Intermolecular Interactions [in Russian], Ch. V. Par. 3.3, Nauka, Moscow (1982).
R. Hellmann, E. Bich, E. Vogel, and V. Vesovic, Intermolecular potential energy surface and thermophysical properties of the CH4–N2 system, J. Chem. Phys., 141, 224301 (10) (2014).
P. V. Novitskii and I. A. Zograf, Estimation of Errors in Measurement Results [in Russian], Énergoatomizdat, Leningrad (1991).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 89, No. 1, pp. 240–249, January–February, 2016.
Rights and permissions
About this article
Cite this article
Fokin, L.R., Kalashnikov, A.N. Transport Properties of a Rarefied Ch4–N2 Gas Mixture. J Eng Phys Thermophy 89, 249–259 (2016). https://doi.org/10.1007/s10891-016-1372-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-016-1372-1