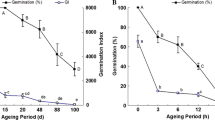
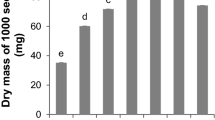
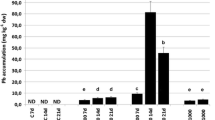
Abstract
Senna obtusifolia L., a common weed in the tropical and subtropical regions of the world, is able to germinate under adverse environmental conditions, suggesting that this species has efficient stress-adaptation strategies. The aims of the present work were to examine the energy metabolism and the antioxidant defense system of the Senna obtusifolia L. during seed germination and initial growth, and the responses to allelochemical-induced stress. Respiratory activity, the activities of alcohol dehydrogenase (ADH), superoxide dismutase (SOD), catalase (CAT),guaicol peroxidase (POD), ascorbate peroxidase (APX), glutathione reductase (GR), lipoxygenase (LOX) and the content of malondialdehyde (MDA) and glutathione (GSSG and GSH) were measured. Shortly after seed imbibition, mitochondrial respiratory activity was active and the presence of SOD, CAT, GR and LOX activity in embryos, along with significant KCN-insensitive respiration, indicated that the production of reactive oxygen species (ROS) is initiated as soon as mitochondrial respiration resumes. Among the fourteen allelochemicals assayed, only coumarin significantly supressed the growth of S. obtusifolia seedlings. Although coumarin reduced the activities of CAT, POD and APX, the GSH, GSSG and MDA levels were not altered. Alpha-pinene, quercetin and ferulic acid did not modify the activity of the antioxidant enzymes or the contents of GSH, GSSH and MDA. Thus the antioxidant defense system of S. obstusifolia may be effective in counteracting the harmful effects of ROS generated during seed germination and initial growth in the presence of toxic allelochemicals.






Similar content being viewed by others
References
Abrahim D, Braguini WL, Kelmer-Bracht AM, Ishii-Iwamoto EL (2000) Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J Chem Ecol 26:611–624. doi:10.1023/A:1005467903297
Abrahim D, Francischini AC, Pergo EM, Kelmer-Bracht AM, Ishii-Iwamoto EL (2003) Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiol Biochem 41:985–991. doi:10.1016/j.plaphy.2003.07.003
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76. doi:10.1016/j.plantsci.2012.07.014
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. doi:10.1104/pp.125.4.1912
Banzatto DA, Kronka SN (2008) Teste de signifiância. In: Banzatto DA, Kronka SN Experimentação Agrícola. FUNEP, 4th edn. Jaboticabal, Brazil, pp 23–52
Blum U (2004) Fate of allelochemicals in soil – the role of soil and rhizosphere microorganism. In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG (eds) Allelopathy. CRC Press, Boca Raton, Chemistry and Mode of Actions of Allelochemicals, pp 57–76
Chiapusio G, Sanchez AM, Reigosa MJ, González L, Pellissier F (1997) Do germination indices adequately reflect allelochemical effects on the germination process? J Chem Ecol 23:2445–2453. doi:10.1023/B:JOEC.0000006658.27633.15
Creel JM, Hoveland CS, Buchanan GA (1968) Germination, growth and ecology of sicklepod. Weed Sci 16:396–400. URL:http://www.Jstor.Org/Stable/4041352
Cruz-Ortega R, Ayala-Cordero G, Anaya AL (2002) Allelochemical stress produced by aqueous leachate of Callicarpa acuminate: effects on roots of bean, maize, and tomato. Physiol Plantarum 116:20–27. doi:10.1034/j.1399-3054.2002.1160103.x
Das SK, Patra JK, Thatoi H (2016) Antioxidative response to abiotic and biotic stresses in mangrove plants: a review. Int Rev Hydrobiol 101:3–19. doi:10.1002/iroh.201401744
Dmitrović S, Simonović A, Mitić N, Savić J, Cingel A, Filipović B, Ninković S (2015) Hairy root exudates of allelopathic weed Chenopodium murale L. induce oxidative stress and down-regulate core cell cycle genes in Arabidopsis and wheat seedlings. Plant Growth Regul 75:365–382. doi:10.1007/s10725-014-9959-z
Dayan FE, Weete JD, Duke SO, Hancock HG (1997) Soybean (Glyxcine max) cultivar differences in response to sulfentrazone. Weed Sci 45:634-641. URL: http://www.jstor.org/stable/4045886
Dayan FE, Weete JD, Hancock HG (1996) Physiological basis for differential sensitivity to sulfentrazone by sicklepod (Senna obstusifolia) and coffee senna (Cassia occientalis). Weed Sci 44:12-17. URL: http://www.jstor.org/stable/4045776
De Gara L (2004) Class III peroxidases and ascorbate metabolism in plants. Phytochem Rev 3:195–205. doi:10.1023/B:PHYT.0000047795.82713.99
DeTullio MC, Arrigoni O (2003) The ascorbic acid system in seeds: to protect and to serve. Seed Sci Res 13:249–260. doi:10.1079/SSR2003143
Devine MD, Duke SO, Fedtke C (1993) Inhibition of amino acid biosynthesis. In: Devine MD, Duke SO, Fedtke C (eds) Physiology of herbicide action, 2nd edn. P T R Prentice Hall, Englewood Cliffs, pp 274–275
Doke N, Miura Y, Chai H-B, Kawakita K (1991) Involvement of active oxygen oxygen in induction of plant defense response against infection and injury. In: Pell E, Steffen K (eds) Active oxygen/oxidative stress and plant metabolism. American Society of Plant Physiologists, Rockville, pp 84–96
Einhellig FA (1993) Mechanism of action of allelochemcials in allelopathy. In. Inderjit, Dakshini KMM, Einhellig FA (eds) allelopathy. Organisms, processes, and applications. ACS symposium series, Washington, DC, pp 96-116
Einhellig FA, Rasmussen JA (1989) Prior cropping with grain sorghum inhibits weeds. J Chem Ecol 15:951–960. doi:10.1007/BF01015190
Eltayeb AE, Yamamoto S, Habora MEE, Matsukubo Y, Aono M, Tsujimoto H, Tanaka K (2010) Greater protection against oxidative damages imposed by various environmental stresses in transgenic potato with higher level of reduced glutathione. Breed Sci 60:101–109. doi:10.1270/jsbbs.60.101
Estabrook RW (1967) Mitochondrial respiratory control and polarographic measurements of ADP/O ratio. Method Enzymol 10:41–47. doi:10.1016/0076-6879(67)10010-4
Ferreira DF (2014) Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciênc Agrotec 38:109–112. doi:10.1590/S1413-70542014000200001
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. doi:10.1007/BF00386001
Geoffroy L, Teisseire H, Couderchet M, Vernet G (2002) Effect of exyfluorfen and diuron alone and in mixture on antioxidative enzymes of Scenedesmus obliquus. Pestic Biochem Physiol 72:178–185. doi:10.1016/S0048-3575(02)00009-3
Giannopolitis CN, Ries SK (1977) Superoxide Dismutases I: occurrence in higher plants. Plant Physiol 59:309–314. doi:10.1104/pp.59.2.309
Golisz A, Lata B, Sw G, Fujii Y (2007) Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol Manag 7:164–171. doi:10.1111/j.1445-6664.2007.00252.x
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acids peroxidation. Arch Biochem Biophys 125:189–198. doi:10.1016/0003-9861(68)90654-1
Holm L, Doll J, Holm E, Pancho J, Herberger J (1997) World weeds: natural histories and distribution, 3rd edn. John Wiley & Sons Inc, New York
Hura T, Dubert F, DąbkowskaT S-RE, Stokłosa A, Lepiarczyk A (2006) Quantitative analysis of phenolics in selected crop species and biological activity of these compounds evaluated by sensitivity of Echinochloa crus-galli. Acta Physiol Plant 28:537–545. doi:10.1007/s11738-006-0049-3
Hussain I, Singh NB, Singh A, Singh H, Singh SC, Yadav V (2017) Exogenous application of phytosynthesized nanoceria to alleviate ferulic acid stress in Solanum lycopersicum. Sci Hortic 214:158-164. Doi: 10/1016/j.Scienta.2016.11.032
Ishii-Iwamoto EL, Abrahim D, Sert MA, Bonato CM, Kelmer-BrachtAM BA (2006) Mitochondria as a site of allelochemical action. In: Reigosa MJ, Pedrol N, González L (eds) Allelopathy: a physiological process with ecological implications. Springer Publishers, Dordrecht, pp 267–284
Jung HI, Kuk YI, Back K, Burgos NR (2008) Resistance pattern and antioxidante enzyme profiles of protoporphyrinogen oxidase (PROTOX) inhibitor-resistant transgenic rice. Pestic Biochem Physiol 91:53–65. doi:10.1016/j.pestbp.2008.01.005
Kanoun-Boulé M, Vicente JAF, Nabais C, Prasad MNV, Freitas H (2009) Ecophlysiological tolerance of duckweeds exposed to copper. Aquat Toxicol 91:1–9. doi:10.1016/j.aquatox.2008.09.009
Kelmer-Bracht AM, Ishii-Iwamoto EL, Suzuki-Kemmelmeier F, Alvarez M, Bracht A (1984) Construction of a liver perfusion apparatus for studies on metabolic regulation and mechanism of drug action. Arquiv Biol Tecnol 27:407–426
Kruidhof HM, Gallandt ER, Haramoto ER, Bastiaans L (2011) Selective weed suppression by cover crop residues: effects of seed mass and timing of species sensitivity. Weed Res 51:177–186. doi:10.1111/j.1365-3180.2010.00825.x
Labouriau LG, Osborn JH (1984) Temperature dependence of the germination of tomato seeds. J Therm Biol 9:285–294. doi:10.1016/0306-4565(84)90010-X
Larkin PJ (1987) Calmodulin levels are not responsible for aluminum tolerance in wheat. Aust J Plant Physiol 14:377–387. doi:10.1071/PP9870377
Lee CY (1982) Alcohol dehydrogenase from Drosophila melanogaster. Methods Enzymol 89:445–450. doi:10.1016/S0076-6879(82)89077-0
Leon RG, Ferrell JA, Sellers BA (2016) Seed production and control of Sicklepod (Senna obtusifolia) and pitted Morningglory (Ipomoea lacunosa) with 2,4-D, Dicamba, and glyphosate combinations. Weed Technol 30:76–84. doi:10.1614/WT-D-15-00108.1
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the folin phenol reagent. J biol Chem 193:265-275. URL: http://www.jbc.org/content/193/1/265.citation
Macias FA (1996) Allelopathy in the serach for natural herbicide models. In. Inderjit, Dakshini KMM, Einhellig FA (eds) allelopathy. Organisms, processes, and applications. ACS symposium series. Washington, DC, pp 310-329
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophy 444:139–158. doi:10.1016/j.abb.2005.10.018
Mahmood K, Khan MB, Song YY, Ye M, Baerson SR, Zeng RS (2013) Differential morphological, cytological and biochemical responses of two rice cultivars to coumarin. Allelopathy J 31:281–296
Mutlu S, Atici Ö, Esim N, Mete E. (2011) Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species Acta Physiol plant 33: 943-951. doi:10.1007/s11738-010-0626-
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. doi:10.1093/oxfordjournals.pcp.a076232
Navrot N, Rouhier N, Gelhaye E, Jacquot JP (2007) Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol Plant 129:185–195. doi:10.1111/j.1399-3054.2006.00777.x
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279. doi:10.1146/annurev.arplant.49.1.249
Norsworthy JK, Oliveira MJ (2006) Sicklepod (Senna obtusifolia) germination and emergence as affected by environmental factors and seeding depth. Weed Sci 54:903–909. doi:10.1614/WS-06-070R2.1
Norsworthy, Jason K., John T. Meehan IV (2005) Herbicidal activity of eight isothiocyanates on Texas panicum (Panicum texanum), large crabgrass (Digitaria sanguinalis), and sicklepod (Senna obtusifolia). Weed Sci 53:515–520. doi: 10.1614/WS-04-208R
Oliveira MJ, Norsworthy JK (2006) Sicklepod (Senna obtusifolia) germination and emergence as affected by environmental factors and seeding depth. Weed Sci 54:903–909. doi:10.1614/WS-06-070R2.1
Pedrol N, González L, Reigosa MJ (2006) Allelopathy and abiotic stress. In: Reigosa MJ, Pedrol N, González L (eds) Allelopathy: a physiological process with ecological implications. Springer Publishers, Dordrecht, pp 171–209
Pereira MRR, Martins CC, Martins D, Silva RJN Da (2014) Saline water stress and the germination of seeds of Raphanus raphanistrum and Senna obtusifolia . Biosci J 30:687–696. URL: http://hdl.handle.net/11449/113234
Pergo EM, Abrahim D, Silva PCS, Kern KA, Silva LJ, Voll E, Ishii-Iwamoto EL (2008) Bidens pilosa L. Exhibits high sensitivity to coumarin in comparison with three other weed species J Chem Ecol 34:499–507. doi:10.1007/s10886-008-9449-8
Pergo EM, Ishii-Iwamoto EL (2011) Changes in energy metabolism and antioxidant defense systems during seeds germination of the weed species Ipomoea triloba L. and the responses to allelochemicals. J Chem Ecol 37:500–513. doi:10.1007/s10886-011-9945-0
Podbielkowska M, Piwocka M, Waszkowska E, Waleza M, Zobel AM (1995) Effect of coumarin and its derivatives on mitosis and ultrastructure of meristematic cells. Int J Pharm 33:7–15. doi:10.3109/13880209509088140
Price AJ, Wilcut JW, Cranmer JR (2004) Physiological behavior of root-absorbed flumioxazin in peanut, ivyleaf morningglory (Ipomoea hederacea), and sicklepod (Senna obtusifolia). Weed Sci 52:718–724. doi:10.1614/WS-04-017R
Puntarullo S, Sanchez RA, Boveris A (1988) Hydrogen peroxide metabolism in soybean embryonic axes at the onset of germination. Plant Physiol 86:626–630. doi:10.1104/pp.86.2.626
Pütter J (1974) Peroxidases. In: Bergmeryer HV (ed) Methods of enzymatic analysis, 2nd edn. Verlag Chemie, Weinheim Academic Press Inc, New York, pp 685–689
Radić S, Babić M, Škobić D, Roje V, Pevalek-Kozlina B (2010) Ecotoxicological effects of aluminum and zinc on growth and antioxidants in Lemna minor L. Ecotoxicol Environ Saf 73:336–342. doi:10.1016/j.ecoenv.2009.10.014
Rizvi SJH, Rizvi V (1992) Exploitation of allelochemicals in improving crop productivity. In: Rizvi SJH, Rizvi V (eds) Allelopathy: basic and applied aspects. Chapman and Hall, London, pp 443–472
Roach T, Colville L, Beckett RP, Minibayeva FV, Havaux M, Kranner I (2015) A proposed interplay between peroxidase, amine oxidase and lipoxygenase in the wounding-induced oxidative busrt in Pisum sativum seedlings. Phytochemistry 112:130–138. doi:10.1016/j.phytochem.2014.06.003
Rolletschek H, Weber H, Borisjuk L (2003) Energy status and is control on embryogenesis of legume. Embryo photosynthesis contributes to oxygen supply and is coupled to biosynthetic fluxes. Plant Physiol 132:1196–1206. doi:10.1104/pp.102.017376
Romero-Romero T, Anaya AL, Cruz-Ortega R (2002) Screening for effects of phytochemical variability on cytoplasmic protein synthesis pattern of crop plants. J Chem Ecol 28:617–629. doi:10.1023/A:1014504531418
Scott A, Knott M (1974) Cluster-analysis method for grouping means in analysis of variance. Biometrics 30:507–512. doi:10.2307/2529204
Siedow JN, Girvin ME (1980) Alternative respiratory pathway. Plant Physiol 65:669–674. doi:10.1104/pp.65.4.669
Siedow JN, Moore AL (1991) The regulation an nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta 1059:121–140. doi:10.1016/S0005-2728(05)80197-5
Singh HP, Batish DR, Kauer S, Arora K, Kohli RK (2006) α-pinene inhibits growth and induces oxidative stress in roots. Ann Bot 98:1261–1269. doi:10.1093/aob/mcl1213
Singh HP, Kauer S, Mittal S, Batish DR, Kohli RK (2009) Essential oil of Artemisia scoparia inhibits plant growth by generating reactive oxygen species and causing oxidative damage. J Chem Ecol 35:154–162. doi:10.1007/s10886-009-9595-7
Smith IK (1985) Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol 79:1044–1047. doi:10.1104/pp.79.4.1044
Svensson SB (1972) The effect of coumarin on growth, production of dry matter, protein and nucleic acids in roots of maize and wheat and the interactions of coumarin with metabolic inhibitors. Physiol Plant 27:13–24. doi:10.1111/j.1399-3054.1972.tb01130.x
Vidal RA, Trezzi MM (2004) Potencial de utilização de cobertura vegetal de sorgo e milheto na supressão de plantas daninhas em condições de campo: II – Efeitos da cobertura morta. Planta Daninha 22:1–10. doi:10.1590/S0100-83582004000100001
Wang H, Xiao X, Yang M, Gao Z, Zang J, Fu X, Chen Y (2014) Effects of salt stress on antioxidant defense system in the root of Kandelia candel. Bot Stud 55:1–7. doi:10.1186/s40529-014-0057-3
Weir TL, Park S-W, Vivanco JM (2004) Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol 7:472–479. doi:10.1016/j.pbi.2004.05.007
Weston LA (1996) Utilization of allelopathy for weed management in agroecosystem. Agron J 88:860–866. doi:10.2134/agronj1996.00021962003600060004x
Whitehead DC (1964) Identification of phydroxybenzoic, vanillic, p-coumaric and ferulic acids in soils. Nature 202:417–418. doi:10.1038/202417a0
Wink M (2010) Annual plant reviews, biochemistry of plant secondary metabolism. Wiley-Blackwell, Chichester
Wojtyla L, Garnczarska M, Zalewski T, Bednarski W, Ratajczak L, Jurga S (2006) A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. J Plant Physiol 163:1207–1220. doi:10.1016/j.jplph.2006.06.014
Acknowledgements
This work was supported by grants from the Fundação Araucária do Estado do Paraná and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Érica Marusa Pergo fellowship holder from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coelho, É.M.P., Barbosa, M.C., Mito, M.S. et al. The Activity of the Antioxidant Defense System of the Weed Species Senna obtusifolia L. and its Resistance to Allelochemical Stress. J Chem Ecol 43, 725–738 (2017). https://doi.org/10.1007/s10886-017-0865-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0865-5