Abstract
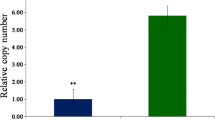
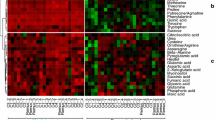
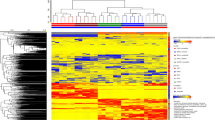
Resistance in Triticeae to fusarium head blight (FHB) is quantitatively inherited. Metabolomics as a tool was used to better understand the mechanisms of resistance and to identify potential FHB resistance biomarker metabolites in barley. Five FHB-resistant two-row barley genotypes (CIho 4196, Zhedar-1, Zhedar-2, Fredrickson, and Harbin-2r) and one FHB-susceptible genotype (CH 9520–30) were each inoculated with either pathogen-suspension or mock-solution. Disease severity, quantified as the proportion of spikelets diseased, varied among genotypes, being the greatest in CH 9520–30. Spikelets were sampled, metabolites extracted with aqueous methanol, and analyzed using an LC-ESI-LTQ-Orbitrap system. A pair wise, resistant vs. susceptible, t-test identified 1774 significant treatment peaks. Canonical discriminant analysis of peak abundance allowed the genotypes to be sorted into three clusters: (i) CH9520-30, (ii) Harbin-2r, (iii) the remaining four genotypes. The t-test was further used to identify resistance-related (RR) and pathogenesis-related (PR) metabolites. The pathogen-produced virulence factor deoxynivalenol (DON), and its detoxification product, DON-3-O-glucoside (D3G) were designated as resistance indicator (RI) metabolites. Metabolites (RR, PR, or RI) occurring in at least two resistant genotypes, showing a two-fold or greater abundance in resistant vs. susceptible lines, and also known to have plant defense functions were selected as potential FHB resistance biomarker metabolites. These included phenylalanine, p-coumaric acid, jasmonate, linolenic acid, total DON produced (TDP), and the proportion of DON converted to D3G (PDC). Total DON was the lowest in CIho 4196, while PDC was the highest in Zhedar-2. The application of RR, PR, and RI metabolites as potential biomarkers to enhance resistance is discussed.



Similar content being viewed by others
References
Agrios, G. N. 2005. Plant Pathology. Elsevier Academic Press, London. p.922.
Bai, G., and Shaner, G. 2004. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 42:135–161.
Buerstmayr, H., Ban, T., and anderson, J. 2009. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breeding 128:1–26.
Boddu, J., Cho, S., Kruger, W. M., and Muehlbauer, G. J. 2006. Transcriptome analysis of the barley–Fusarium graminearum interaction. Mol. Plant-Microbe Interact.19: 407–417.
Bollina, V., Kumaraswamy, G. K., Kushalappa, A. C., Choo, T. M., Dion, Y., Rioux, S., Faubert, D., and Hamzehzarghani, H. 2010. Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against Fusarium head blight. Mol. Plant Pathol.11:769–782.
Boutigny, A., Barreau, C., Atanasova-Penichon, V., Verdal-Bonnin, M., Pinson-Gadais, L., and Richard-Forget, F. 2009. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 113:746–753.
Choo T. M. 2006. Breeding barley for resistance to fusarium head blight and mycotoxin accumulation. Plant Breed Rev. 26:125–169.
Choo T. M., Vigier B., Chen Q. Q., Martin R. A., Ho K. M., and Savard M. 2004. Barley traits associated with resistance to fusarium head blight and deoxynivalenol accumulation. Phytopathology 94:1145–1150.
Desjardins, A. E. 2006. Fusarium Mycotoxins: Chemistry, Genetics and Biology, APS Press, St. Paul Minnesota, USA.
Dixon, R. A. 2001. Natural products and plant disease resistance. Nature 411:843–847.
Geddes, J., Eudes, F., Laroch, A., and Selinger, L. B. 2008. Differential expression of proteins in response to the interaction between the pathogen Fusarium graminearum and its host, Hordeum vulgare. Proteomics 8:545–554.
Gilbert, J. and Tekauz, A. 2000. Review: Recent developments in research on fusarium head blight of wheat in Canada. Can. J. Plant Pathol. 22:1–8.
Golkari, S., Gilbert, J., Prasher, S., and Procunier, J. D. 2007. Microarray analysis of Fusarium graminearum-induced wheat genes: identification of organ specific and differentially expressed genes. Plant Biotechnol. J. 5:38–49.
Hamzehzarghani, H., Paranidharan, V., Abu-Nada, Y., Kushalappa, A. C., Dion, Y., Rioux, S., Comeau, A., Yaylayan, V., and Marshall, W. 2008. Metabolic profiling coupled with statistical analyses for potential high throughput screening of quantitative resistance to fusarium head blight in wheat cultivars. Can. J. Plant Pathol.30:24–36.
Jansen, C., Von-Wettstein, D., Schäfer, W., Kogel, K., Felk, A., and Maier, F. 2005. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 102:16892–16897.
Jia, H., Cho, S. and Muehlbauer, G. 2009. Transcriptome analysis of a wheat near-isogenic line pair carrying Fusarium head blight-resistant and-susceptible alleles. Mol. Plant-Microbe Interact. 22:1366–1378.
Kumaraswamy, G. K., Bollina, V., Kushalappa, A. C., Choo, T. M., Dion, Y., Rioux, S., Mamer, O., and Faubert, D. 2011. Metabolomics technology to phenotype resistance in barley against Gibberella zeae. Euro. J. Plant Pathol. 130:29–43.
Lemmens, M., Scholz, U., Berthiller, F., dall’asta, C., Koutnik, A., Schuhmacher, R., Adam, G., Buerstmayr, H., Mesterházy, Á., and Krska, R. 2005. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact.18:1318–1324.
Li, G. and Yen, Y. 2008. Jasmonate and ethylene signaling pathway may mediate Fusarium head blight resistance in wheat. Crop Sci. 48:888–896.
Ma, S. and Bohnert, H. 2007. Integration of Arabidopsis thaliana stress-related transcript profiles, promoter structures, and cell-specific expression. Genome Biol. 8:R49.
Mckeehen, J. D., Busch, R. H., and Fulcher, R. G. 1999. Evaluation of wheat (Triticum aestivum L.) phenolic acids during grain development and their contribution to Fusarium resistance. J. Agric. Food Chem. 47:1476–1482.
Miller, J. D., Young, J. C., and Arnison, P. G. 1986. Detoxification of deoxynivalenol by suspension cultures of a Fusarium head blight resistant wheat cultivar. Can. J. Plant Pathol. 8:147–50.
Proctor, R. H., Hohn, T. M., and Mccormick, S. P. 1995. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 8:593–601.
Robert-Seilaniantz, A., Navarro, L., Bari, R., and Jones, J. D. G. 2007. Pathological hormone imbalances. Curr. Opin. Plant Biol. 10:372–379.
Shah, J. 2009. Plants under attack: systemic signals in defense. Curr. Opin. Plant Biol. 12:459–464.
Steiner, B., Kurz, H., Lemmens, M., and Buerstmayr, H. 2009. Differential gene expression of related wheat lines with contrasting levels of head blight resistance after Fusarium graminearum inoculation. Theor. Appl. Genet. 118:753–764.
Tautenhahn, R., Böttcher, C., and Neumann. S. 2007. Annotation of LC/ESI-MS mass signals. Proceedings of BIRD 2007 - 1st International Conference on Bioinformatics Research and Development, Springer LNBI 4414.
Tekauz, A., Mccallum, B., and Gilbert, J. 2000. Review: Fusarium head blight of barley in western Canada. Can. J. Plant Pathol. 22:9–16.
Vorst, O., De Vos, C. H. R., Lommen, A., Staps, R. V., Visser, R. G. F., Bino, R. J., and Hall, R. D. 2005. A non-directed approach to the differential analysis of multiple LC-MS derived metabolic profiles. Metabolomics 1:169–180.
Yoshida, M., Kawada, N., and Nakajima, T. 2007. Effect of infection timing on Fusarium head blight and mycotoxin accumulation in open-and closed-flowering barley. Phytopathology 97:1054–1062.
Zadoks, J. C., Chang, T. T., and Konzak, B. F. 1974. A decimal code for the growth stages of cereals. Weed Res. 14:415–421.
Zhou, W., Eudes, F., and Laroche, A. 2006. Identification of differentially regulated proteins in response to a compatible interaction between the pathogen Fusarium graminearum and its host, Triticum aestivum. Proteomics 6:4599–4609.
Acknowledgements
This project was funded by the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ), Centre de recherche sur les grains inc. (CEROM), and the Fédération des producteurs de porc du Québec (FPPQ), Québec, Canada.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Phenylpropanoid, flavonoid and fatty acid RR metabolites identified in five Fusarium head blight (FHB) resistant barley genotypes, as compared to susceptible CH 9520–30 (DOC 80 kb)
Supplementary Table S2
Fragmentation patterns of biomarkers identified in barley genotypes against Fusarium head blight (FHB) (DOC 37 kb)
Figure S1
DON and its adduct, DON + CH3COO−, 3ADON and its adduct 3ADON + CH3COO−, and DON-3-O-glucoside (D3G) and and its adduct, D3G + CH3COO-, each of the three at one retention time (RT) (PPT 677 kb)
Figure S2
Abundances of DON, D3G, and 3ADON and their adducts in barley based on LC-LTQ-Orbitrap: a) Abundances of DON and DON + CH3COO−; b) Abundances of D3G and D3G + CH3COO−; c) Abundances of 3−ADON and 3-ADON + CH3COO− in lemma + palea of six barley genotypes inoculated with F. graminearum under greenhouse conditions, based on LC-ESI-LTQ-Orbitrap analysis (PPT 130 kb)
Rights and permissions
About this article
Cite this article
Kumaraswamy, K.G., Kushalappa, A.C., Choo, T.M. et al. Mass Spectrometry Based Metabolomics to Identify Potential Biomarkers for Resistance in Barley against Fusarium Head Blight (Fusarium graminearum). J Chem Ecol 37, 846–856 (2011). https://doi.org/10.1007/s10886-011-9989-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9989-1