Abstract
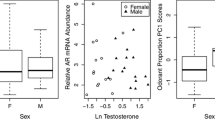
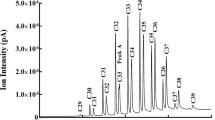
Songbird preen oil contains volatile and semivolatile compounds that may contain information about species, sex, individual identity, and season. We examined the relationship between testosterone (T) and the amounts of preen oil volatile and semivolatile compounds in wild and captive dark-eyed juncos (Junco hyemalis). In wild males and females, we observed an increase in volatile compound relative concentration early in the breeding season. This increase mirrored previously described seasonal elevation in T levels in wild males and females, suggesting a positive relationship between hormone levels and preen gland secretions, and a possible role for these secretions in signaling receptivity. In females, the greatest relative concentrations of most compounds were observed close to egg laying, a time when steroid hormones are high and also the only time that females respond to an injection of gonadotropin-releasing hormone with a short-term increase in T. In a study of captive juncos held on short days, we asked whether the seasonal increases observed in the wild could be induced with experimental elevation of T alone. We found that exogenous T stimulated the production of some volatile compounds in non-breeding individuals of both sexes. However, of the 15 compounds known to increase during the breeding season, only four showed an increase in relative concentration in birds that received T implants. Our results suggest that testosterone levels likely interact with other seasonally induced physiological changes to affect volatile compound amounts in preen oil.





Similar content being viewed by others
References
Abalain, J. H., Amet, Y., Daniel, J. Y., Floch, H. H. 1983. Testosterone stimulation of DNA-dependent DNA polymerase activities in the cloacal and uropygial glands of the male quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 52:164-171.
Adkins-Regan, E. 1981. Hormone specificity, androgen metabolism, and social behavior. Am. Zool. 21:257-271.
Amet, Y., Abalain, J. H., Daniel, J. Y., Di Stefano, S., Floch, H. H. 1986. Testosterone regulation of androgen receptor levels in the uropygial gland of quails (Coturnix coturnix): a further proof for the androgen dependency of the uropygial gland. Gen. Comp. Endocrinol. 62:210-216.
Balthazart, J., Taziaux, M. 2009. The underestimated role of olfaction in avian reproduction? Behav. Brain Res. 200:248-259.
Bentley, G. E. 2001. Unraveling the enigma: the role of melatonin in seasonal processes in birds. Microscopy Res. Tech. 53:63-71.
Bentley, G. E., Van’t Hof, T., Ball, G. F. 1999. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc. Natl. Acad. Sci. USA 96:4674-4679.
Bohnet, S., Rogers, L., Sasaki, G., Kolattukudy, P. E. 1991. Estradiol induces proliferation of peroxisome-like microbodies and the production of 3-hydroxy fatty acid diesters, the female pheromones, in the uropygial glands of male and female mallards. J. Biol. Chem. 266:9795-9804.
Bonadonna, F., Mardon, J. 2010. One house two families: petrel squatters get a sniff of low-cost breeding opportunities. Ethology 116:176-182.
Bonadonna, F., Miguel, E., Grosbois, V., Jouventin, P., Bessiere, J.-M. 2007. Individual odor recognition in birds: an endogenous olfactory signature on petrel’s feathers? J. Chem. Ecol. 33:1819-1829.
Clotfelter, E. D., O’neal, D. M., Gaudioso, J. M., Casto, J. M., Parker-Renga, I. M., Snajdr, E., Duffy, D. L., Nolan, V., Jr., Ketterson, E. D. 2004. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Hormones Behav. 46:171-178.
Daniel, J. Y., Vignon, F., Assenmacher, I., Rochefort, H. 1977. Evidence of androgen and estrogen receptors in the preen gland of male ducks. Steroids 30:703-709.
Douglas III, H. D., Kitaysky, A. S., Kitaiskaia, E. V. 2008. Seasonal variation in progesterone and odorant emissions among breeding crested auklets (Aethia cristatella). Hormones Behav.54:325-329.
Edwards, A., Jones, S. M., Davies, N. W. 2005. Patterns of peripheral steroid metabolism vary with sex, season, and tissue type in blotched blue-tongued lizards (Tiliqua nigrolutea). Gen. Comp. Endocrinol. 140:14-24.
Farner, D. S., Wingfield, J. C. 1980. Reproductive endocrinology of birds. Annu. Rev. Physiol. 42:457-472.
Griffiths, R., Double, M. C., Orr, K., Dawson, R. J. G. 1998. A DNA test to sex most birds. Molec. Ecol. 7:1071-1075.
Hirao, A., Aoyama, M., Sugita, S. 2009. The role of uropygial gland on sexual behavior in domestic chicken Gallus gallus domesticus. Behav. Processes 80:115-120.
Jacob, J. P., Ziswiler, V. 1982. The uropygial gland. pp 199-324 in: Farner D. S., King J. R., Parkes K. C., (eds.). Avian Biology. New York: Academic Press.
Jawor, J. M., Mcglothlin, J. W., Casto, J. M., Greives, T. J., Snajdr, E. A., Bentley, G. E., Ketterson, E. D. 2006. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis). Gen. Comp. Endocrinol. 149:182-189.
Jawor, J. M., Mcglothlin, J. W., Casto, J. M., Greives, T. J., Snajdr, E. A., Bentley, G. E., Ketterson, E. D. 2007. Testosterone response to GnRH in a female songbird varies with stage of reproduction: implications for adult behaviour and maternal effects. Funct. Ecol. 21:767-775.
Ketterson, E. D., Nolan, V., Jr., Wolf, L., Ziegenfus, C., Dufty, A. M., Ball, G. F., Johnsen, T. S. 1991. Testosterone and avian life histories: the effect of experimentally elevated testosterone on corticosterone and body mass in dark-eyed juncos. Hormones Behav. 25:489-503.
Ketterson, E. D., Nolan, V., Jr., Wolf, W. L., Ziegenfus, C. 1992. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Amer. Nat. 140:980-990.
Ketterson, E. D., Nolan, V., Jr., Casto, J. M., Buerkle, C. A., Clotfelter, E. D., Grindstaff, J. L., Jones, K. J., Lipar, J. L., Mcnabb, F. M. A., Neudorf, D. L. H., Parker-Renga, I. M., Schoech, S. J., Snajdr, E. 2001. Testosterone, phenotype, and fitness: a research program in evolutionary behavioral endocrinology. pp 19-40 in: Dawson A., Chaturvedi C. M., (eds.). Avian Endocrinology. New Delhi, India: Narosa Publishing House.
Ketterson, E. D., Nolan, V., Jr., Sandell, M. 2005. Testosterone in females: mediator of adaptive traits, constraint on the evolution of sexual dimorphism, or both? The Amer. Nat.166:S85-S98.
Maiti, B. R., Ghosh, A. 1969. Effect of cortisone on mitotic activity and cell loss in the uropygial gland of male pigeons. Acta Anatomica 74:97-103.
Maiti, B. R., Ghosh, A. 1972. Probable role of androgen in the regulation of the uropygial gland. Gen. Comp. Endocrinol. 19:527-536.
Mardon, J., Saunders, S. M., Anderson, M. J., Couchoux, C., Bonadonna, F. 2010. Species, gender, and identity: cracking petrels’ sociochemical code. Chem. Senses 35:309-321.
Massa, R. 1984. Patterns and biological significance of steroidal hormone metabolism in birds. J. Exp. Zool. 232:531-537.
Nolan, V., Jr., Ketterson, E. D., Cristol, D. A., Rogers, C. M., Clotfelter, E. D., Titus, R., Schoech, S. J., Snajdr, E. 2002. Dark-eyed Junco (Junco hyemalis). Poole A., Gill F., (eds.). Philadelphia, PA: The Birds of North America, Inc.
Novotny, M. V., Harvey, S., Jemiolo, B. 1990. Chemistry of male dominance in the house mouse, Mus domesticus. Experientia (Basel) 46:109-113.
Ponmanickam, P., Palanivelu, K., Govindaraj, S., Baburajendran, R., Habara, Y., Archunan, G. 2010. Identification of testosterone-dependent volatile compounds and proteins in the preputial gland of rat Rattus norvegicus. Gen. Comp. Endocrinol. 167:35-43.
Riters, L. V., Baillien, M., Eens, M., Pinxten, R., Foidart, A., Ball, G. F., Balthazart, J. 2001. Seasonal variation in androgen-metabolizing enzymes in the diencephalon and the telencephalon of the male European starling (Sturnus vulgaris ). J. Neuroendocrinol. 13:985-997.
Shanbhag, B. A., Sharp, P. J. 1996. Immunocytochemical localization of androgen receptor in the comb, uropygial gland, testis, and epididymis in the domestic chicken. Gen. Comp. Endocrinol. 101:76-82.
Shaw, C. L., Rutter, J. E., Austin, A. L., Garvin, M. C., Whelan, R. J. 2011. Volatile and semivolatile compounds in gray catbird uropygial secretions vary with age and between breeding and wintering grounds. J. Chem. Ecol. 37:329-339.
Soini, H. A., Schrock, S. E., Bruce, K. E., Wiesler, D., Ketterson, E. D., Novotny, M. V. 2007. Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J. Chem. Ecol.33:183-198.
Soma, K. K., Schlinger, B. A., Wingfield, J. C., Saldanha, C. J. 2003. Brain aromatase, 5α-reductase, and 5β-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. Development. Neurobiol. 56:209-221.
Tamarkin, L., Baird, C. J., Almeida, O. F. X. 1985. Melatonin: a coordinating signal for mammalian reproduction? Science 227:714-720.
Watanabe, T., Yamamura, T., Watanabe, M., Yasuo, S., Nakao, N., Dawson, A., Ebihara, S., Yoshimura, T. 2007. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am. J. Physiol. Reg. Integ. Comp. Physiol. 292:R658-R572.
Whelan, R. J., Levin, T. C., Owen, J. C., Garvin, M. C. 2010. Short-chain carboxylic acids from gray catbird (Dumetella carolinensis) uropygial secretions vary with testosterone levels and photoperiod. Comp. Biochem. Physiol. 156:183-188.
Whittaker, D. J., Soini, H. A., Atwell, J. W., Hollars, C., Novotny, M. V., Ketterson, E. D. 2010. Songbird chemosignals: preen oil volatile compounds vary among individuals, sexes, and populations. Behav. Ecol.21:608-614.
Whittaker, D. J., Richmond, K. M., Miller, A. K., Kiley, R., Bergeon Burns, C., Atwell, J. W., Ketterson, E. D. 2011. Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav. Ecol. 22:1256-1263.
Winkler, S. M., Wade, J. 1998. Aromatase activity and regulation of sexual behaviors in the green anole lizard. Physiol. Behav. 64:723-731.
Wyatt, T. D. 2003. Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge: Cambridge University Press.
Acknowledgments
We thank Ryan Kiley, Christine Bergeon Burns, Kim Rosvall, and Mark Peterson for assistance in capturing juncos and maintaining them in captivity. Thanks to Jim Goodson for lending equipment and Kevin McLane for help processing samples. We thank Greg Demas for comments on an earlier draft. All work was conducted in compliance with the Bloomington Institutional Animal Care and Use Committee guidelines (BIACUC protocol 09-037) and with permission from the US Department of Fish and Wildlife, the Virginia Department of Game and Inland Fisheries, and the US Forest Service. This research was supported by the Indiana Academy of Sciences, and the Indiana University Faculty Research Support Program. Chemical analysis was sponsored jointly by the METACyt Initiative of Indiana University, a major grant from the Lilly Endowment, Inc., and the Lilly Chemistry Alumni Chair funds (to M.V.N.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whittaker, D.J., Soini, H.A., Gerlach, N.M. et al. Role of Testosterone in Stimulating Seasonal Changes in a Potential Avian Chemosignal. J Chem Ecol 37, 1349–1357 (2011). https://doi.org/10.1007/s10886-011-0050-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-0050-1