Abstract
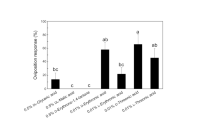
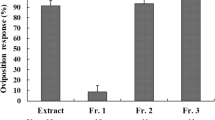
The banded sunflower moth (BSFM), Cochylis hospes Walshingham (Lepidoptera: Cochylidae) is a specialist insect, the larvae of which feed on sunflowers, Helianthus spp., and a few other species of Compositae. It is one of the most important pests of sunflower in the USA. Previous work on H. annuus, the cultivated sunflower, revealed two diterpenoids that function as oviposition stimulants for female BSFM, and that other, more polar compounds also stimulated oviposition. Using a bioassay-guided approach, we isolated three additional diterpenoids, grandifloric acid (1), 15β-hydroxy-ent-trachyloban-19-oic acid (2), and 17-hydroxy-16α-ent-kauran-19-oic acid (3), from polar fractions of pre-bloom sunflower head extracts. In laboratory bioassays, purified natural samples of each of these compounds stimulated oviposition by female BSFM. Structure–activity relationships of the five diterpenoids known to stimulate oviposition by female BSFM are discussed.





Similar content being viewed by others
References
Barker, J. F. 1988. Laboratory rearing of the banded sunflower moth, Cochylis hospes (Lepidoptera: Cochylidae). J. Kans. Entomol. Soc. 61:350–352.
Barker, J. F. 1997. Oviposition by the banded sunflower moth (Lepidoptera: Cochylidae) in response to constituents of the bracts and leaves of Helianthus annuus. J. Econ. Entomol. 90:160–164.
Beale, M. H., Bearder, J. R., Macmillan, J., Matsuo, A., and Phinney, B. O. 1983. Diterpene acids from Helianthus species and their microbiological conversion by Gibberella fujikuroi, mutant B1–41a. Phytochemistry 22:875–881.
Bjeldanes, L. F., and Geissman, T. A. 1972. Constituents of Helianthus ciliaris. Phytochemistry. 11:327–332.
Bohlmann, F., Kramp, W., Jakupovic, J., Robinson, H., and King, R. M. 1982. Diterpenes from Baccharis species. Phytochemistry. 21:399–403.
Charlet, L. D., Brewer, G. J., and Franzmann, B. A. 1997. Sunflower insects, pp. 183–261, in A. A. Schneiter (ed.). Sunflower Technology and Production American Society of Agronomy, Madison, WI USA.
Coates, R. M., Denissen, J. F., Juvik, J. A., and Babka, B. A. 1988. Identification of α-santalenoic and endo-β-bergamotenoic acids as moth oviposition stimulants from wild tomato leaves. J. Org. Chem. 53:2186–2192.
Connolly, J. D. and Hill, H. A. 1991. Dictionary of terpenoids, vol. 2, Di- and Higher Terpenoids, pp. 914–969. Chapman and Hall, London and New York.
Etse, J. T., Gray, A. I., and Waterman, P. G. 1987. Chemistry in the Annonaceae, XXIV. Kaurane and Kaur-16-ene Diterpenes from the Stem Bark of Annona reticulata. J. Nat. Prod. 50:979–983.
Ferguson, G., McCrindle, R., Murphy, S. T., and Parvez, M. 1982. Further diterpenoid constituents of Helianthus annuus L Crystal and molecular structure of methyl ent-15β-hydroxy-trachyloban-19-oate. J. Chem. Res. 200–201.
Foster, S. P., Noll, M., Grugel, S., and Charlet, L. D. 2003. A reinvestigation of the role of sunflower chemicals in host selection by female banded sunflower moth, Cochylis hospes (Walshingham) (Lepidoptera: Tortricidae). J. Kans. Entomol. Soc. 76:387–396.
Gershenzon, J., Ohno, N., and Mabry, T. J. 1981. The terpenoid chemistry of sunflowers (Helianthus). Revista Latinoamericana de Quimica 12:53–61.
Hanson, J. R., Siverns, M., Piozzi, F., and Savona, G. 1976. The 13C nuclear magnetic resonance spectra of kauranoid diterpenes. J. Chem. Soc. Perkin 1:114–117.
Harrigan, G. G., Da, S., Bolzani, V., Gunatilaka, A. A. L., and Kingston, D. G. I. 1994. Kaurane and trachylobane diterpenes from Xylopia aethiopica. Phytochemistry 36:109–113.
Herz, W., and Kulanthaivel, P. 1983. ent-Kauranes and Trachylobanes from Helianthus radula. Phytochemistry 22:2543–2546.
Herz, W., Govindan, S. V., and Watanabe, K. 1982. Diterpenes of Helianthus rigidus and H. salicifolius. Phytochemistry 21:946–947.
Herz, W., Kulanthaivel, P., and Watanabe, K. 1983. ent-Kauranes and other constituents of three Helianthus species. Phytochemistry 22:2021–2025.
Jackson, D. M., Severson, R. F., Johnson, A. W., and Herzog, G. A. 1986. Effects of cuticular duvane diterpenes from green tobacco leaves on tobacco budworm (Lepidoptera: Noctuidae) oviposition. J. Chem. Ecol. 12:1349–1359.
Jackson, D. M., Severson, R. F., Sisson, V. A., and Stephenson, M. G. 1991. Ovipositional response of the tobacco budworm moths (Lepidoptera: Noctuidae) to cuticular labdanes and sucrose esters from the green leaves of Nicotiana glutinosa L. (Solinaceae). J. Chem. Ecol. 17:2489–2506.
Jefferies, P. R., and Payne, T. G. 1965. Chemistry of Euphorbiaceae. XII. Compounds derived from a new Beyeria species. Aust. J. Chem. 18:1441–1450.
Melek, F. R., Gage, D. A., Gershenzon, J., and Mabry, T. J. 1985. Sesquiterpene lactone and diterpene constituents of Helianthus annuus. Phytochemistry 24:1537–1539.
Mitscher, L. A., Rao, G. S. R., Veysoglu, T., Drake, S., and Haas, T. 1983. Isolation and identification of trachyloban-19-oic and (−)-kaur-16-en-19-oic acids as antimicrobial agents from the prairie sunflower, Helianthus annuus. J. Nat. Prod. 46:745–746.
Moreno, B., Delle monache, G., Delle monache, F., and Marini-Bettolo, G. B. 1980. Flavones and kauranoid diterpenes from Eupatorium tinifolium H.B.K. Researches on plant antitumor principles. Farmaco Ed. Sci. 35:457–464.
Morris, B. D., Foster, S. P., Grugel, S., and Charlet, L. D. 2005. Isolation of the diterpenoids, ent-kauran-16α-ol and ent-atisan-16α-ol, from sunflowers, as oviposition stimulants for the banded sunflower moth, Cochylis hospes. J. Chem. Ecol. 31:89–103.
Mullin, C. A., Alfatafta, A. A., Harman, J. L., Everett, S. L., and Serino, A. A. 1991. Feeding and toxic effects of floral sesquiterpene lactones, diterpenes, and phenolics from sunflower (Helianthus annuus L.) on western corn rootworm. J. Agric. Food Chem. 39:2293–2299.
Ohno, N., and Mabry, T. J. 1980. Sesquiterpene lactones and diterpene carboxylic acids in Helianthus niveus ssp. canescens. Phytochemistry 19:609–614.
Ohno, N., Mabry, T. J., Zabel, V., and Watson, W. H. 1979. Tetrachyrin, a new rearranged kaurenoid lactone, and diterpene acids from Tetrachyron orizabaensis and Helianthus debilis. Phytochemistry 18:1687–1689.
Schneiter, A. A., and Miller, J. F. 1981. Description of sunflower growth stages. Crop Science 21:901–903.
Toyota, M., Wada, T., and Ihara, M. 2000. Total syntheses of (−)-methyl atis-16-en-19-oate, (−)-methyl kaur-16-en-19-oate, and (−)-methyl trachyloban-19-oate by a combination of palladium-catalyzed cycloalkenylation and homoallyl-homoallyl radical rearrangement. J. Org. Chem. 65:4565–4570.
Watanabe, K., Ohno, N., Yoshioka, H., Gershenzon, J., and Mabry, T. J. 1982. Sesquiterpene lactones and diterpenoids from Helianthus argophyllus. Phytochemistry 21:709–713.
Wu, Y.-C., Hung, Y.-C., Chang, F.-R., Cosentino, M., Wang, H.-K., and Lee, K.-H. 1996. Identification of ent-16β, 17-dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J. Nat. Prod. 59:635–637.
Acknowledgements
We thank Sharon Grugel for supplying insects and Kirk Anderson for assistance in growing sunflowers. This work was partially supported by a grant from the National Sunflower Association and North Dakota SBARE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, B.D., Charlet, L.D. & Foster, S.P. Isolation of Three Diterpenoid Acids from Sunflowers, as Oviposition Stimulants for the Banded Sunflower Moth, Cochylis hospes . J Chem Ecol 35, 50–57 (2009). https://doi.org/10.1007/s10886-008-9567-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9567-3