Abstract
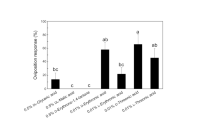
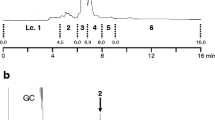
Two diterpenoid alcohols, ent-kauran-16α-ol (1) and ent-atisan-16α-ol (2), were isolated from pre bloom (R3-R4 stage) sunflower heads as oviposition stimulants for the banded sunflower moth, Cochylis hospes. Fractionation of a sunflower head extract, by normal-phase flash column chromatography, resulted in an early eluting fraction exhibiting significant activity in an egg-laying bioassay. Compounds 1 and 2, along with ent-trachyloban- 19-oic acid (3) and ent-kaur-16-en-19-oic acid (4), were isolated as the major components of this fraction and identified by their NMR and mass spectra. The purified compounds were individually tested for ovipositional activity in dose-response bioassays. In these bioassays, compounds 1 and 2 gave linear dose responses, with increasing numbers of eggs laid as the dosage of either increased. Compounds 3 and 4 failed to stimulate significant egg-laying at any of the dosages tested. A factorial design bioassay, using compounds 1 and 2, showed that 1 was relatively more stimulatory than 2, and that there was no synergistic effect on oviposition when the two compounds were combined.
Similar content being viewed by others
References
Attygalle, A. B. 1998. Microchemical techniques. pp. 207–294. J. G. Millar and C. F. Haynes (eds.). Methods in Chemical Ecology, Vol. 1, Chemical Methods. Kluwer Academic Publishers, Boston, USA.
Barker, J. F. 1988. Laboratory rearing of the banded sunflower moth, Cochylis hospes (Lepidoptera: Cochylidae). J. Ks. Entomol. Soc. 61:350–352.
Barker, J. F. 1997. Oviposition by the banded sunflower moth (Lepidoptera: Cochylidae) in response to constituents of the bracts and leaves of Helianthus annuus. J. Econ. Entomol. 90:160–164.
Beale, M. H., Bearder, J. R., Macmillan, J., Matsuo, A., and Phinney, B. O. 1983. Diterpene acids from Helianthus species and their microbiological conversion by Gibberella fujikuroi, mutant B1-41a. Phytochemistry 22:875–881.
Breeden, D. C., Young, T. E., Coates, R. M., and J uvik, J. A. 1996. Identification and bioassay of kairomones for Helicoverpa zea. J. Chem. Ecol. 22:513–539.
Charlet, L. D., Brewer, G. J., and Franzmann, B. A. 1997. Sunflower insects, pp. 183–261, A. A. Schneiter (ed.). Sunflower Technology and Production. American Society of Agronomy, Madison, WI, USA.
Faulkner, D. F., Lebby, V., and Waterman, P. G. 1985. Further diterpenes from stem bark of Xylopia aethiopica. Planta Med. 51:354–355.
Foster, S. P., Noll, M., Grugel, S., and Charlet, L. D. 2003. A reinvestigation of the role of sunflower chemicals in host selection by female banded sunflower moth, Cochylis hospes (Walshingham) (Lepidoptera: Tortricidae). J. Ks. Entomol. Soc. 76:387–396.
Herz, W., Govindan, S. V., and Watanabe, K. 1982. Diterpenes of Helianthus rigidus andH. salicifolius. Phytochemistry 21:946–947.
Herz, W., Kulanthaivel, P., and Watanabe, K. 1983. ent-Kauranes and other constituents of three Helianthusspecies. Phytochemistry 22:2021–2025.
Kalinovsky, A. I., Serebryakov, E. P., Zolotarev, B. M., Simolin, A. V., Kucherov, V. F., and Chizhov, O. S. 1970. Mass spectra of kaurene derivatives-I. The mass spectra of some (−)-kauran-16-ols. Org. Mass Spect. 3:1393–1400.
Kanno, H. and Harris, M. O. 2000. Physical features of grass leaves influence the placement of eggs within the plant by the Hessian fly. Entomol. Exp. Appl. 96:69–80.
Leong, Y. W. and Harrison, L. J. 1997. ent-Trachylobane diterpenes from the liverwort Mastigophora diclados. Phytochemistry 45:1457–1459.
Mitscher, L. A., Rao, G. S. R., Veysoglu, T., Drake, S., and Haas, T. 1983. Isolation and identification of trachyloban-19-oic and (−)-kaur-16-en-19-oic acids as antimicrobial agents from the prairie sunflower, Helianthus annuus. J. Nat. Prod. 46:745–746.
Morris, B. D., Foster, S. P., and Harris, M. O. 2000. Identification of 1-octacosanal and 6-methoxy-2-benzoxazolinone from wheat as ovipositional stimulants for Hessian fly, Mayetiola destructor. J. Chem. Ecol. 26:859–873.
Ohno, N., Mabry, T. J., Zabel, V., and Watson, W. H. 1979. Tetrachyrin, a new rearranged kaurenoid lactone, and diterpene acids from Tetrachyron orizabaensis and Helianthus debilis. Phytochemistry 18:1687–1689.
Spencer, J. L. 1996. Waxes enhance Plutella xylostella oviposition response to sinigrin and cabbage homogenates. Entomol. Exp. Appl. 81:165–173.
St. Pyrek, J. 1970. New pentacyclic diterpene acid trachyloban-19-oic acid from sunflower. Tetrahedron 26:5029–5032.
St. Pyrek, J. 1984. Neutral diterpenoids of Helianthus annuus. J. Nat. Prod. 47:822–827.
Toyota, M., Wada, T., Fukumoto, K., and Ihara, M. 1998. Total synthesis of (±)-methyl atis-16-en-19-oate via homoallyl-homoallyl radical rearrangement. J. Am. Chem. Soc. 120:4916–4925.
Toyota, M., Wada, T., and Ihara, M. 2000. Total syntheses of (—)-methyl atis-16-en-19-oate, (—)-methyl kaur-16-en-19-oate, and (—)-methyl trachyloban-19-oate by a combination of palladium-catalyzed cycloalkenylation and homoallyl-homoallyl radical rearrangement. J. Org. Chem. 65:4565–4570.
Watanabe, K., Ohno, N., Yoshioka, H., Gershenzon, J., and Mabry, T. J. 1982. Sesquiterpene lactones and diterpenoids from Helianthus argophyllus. Phytochemistry 21:709–713.
Wu, Y. C., Hung, Y. C., Chang, F. R., Cosentino, M., Wang, H. K., and Lee, K. H. 1996. Identification of ent-16β, 17-dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J. Nat. Prod. 59:635–637.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MORRIS, B.D., FOSTER, S.P., GRUGEL, S. et al. ISOLATION OF THE DITERPENOIDS, ENT-KAURAN-16α-OL AND ENT-ATISAN-16α-OL, FROM SUNFLOWERS, AS OVIPOSITION STIMULANTS FOR THE BANDED SUNFLOWER MOTH, Cochylis hospes. J Chem Ecol 31, 89–102 (2005). https://doi.org/10.1007/s10886-005-0976-2
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-0976-2