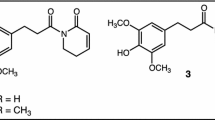
Abstract
The detoxification limitation hypothesis is firmly entrenched in the literature to explain various aspects of the interaction between herbivores and plant toxins. These include explanations for the existence of specialist and generalist herbivores and for the prevalence of each of these. The hypothesis suggests that the ability of mammalian herbivores to eliminate plant secondary metabolites (PSMs) largely determines which plants, and how much, they can eat. The value of the hypothesis is that it provides a clear framework for understanding how plant toxins might limit diet breadth. Thus, it is surprising, given its popularity, that there are few studies that provide experimental support either for or against the detoxification limitation hypothesis. There are two likely reasons for this. First, Freeland and Janzen did not formally propose the hypothesis, although it is implicit in their paper. Second, it is a difficult hypothesis to test, requiring an understanding of the metabolic pathways that lead to toxin elimination. Recent attempts to test the hypothesis appear promising. Results suggest that herbivores can recognize mounting saturation of a detoxification pathway and adjust their feeding accordingly to avoid intoxication. One strategy they use is to ingest a food containing a toxin that is metabolized by a different pathway. This demonstrates that careful selection of food plants is a key to existing in a chemically complex environment. As more studies characterize the detoxification products of PSMs, we will better understand how widespread this phenomenon is.



Similar content being viewed by others
References
Aldrich, C. G., Rhodes, M. T., Miner, J. L., Kerley, M. S., and Paterson, J. A. 1993. The effects of endophyte-infected tall fescue consumption and use of a dopamine antagonist on intake, digestibility, body temperature, and blood constituents in sheep. Anim. Sci. 71:158–163.
Allen, C. T., Peden-Adams, M. M., Eudaly, J., and Keil, D. E. 2003. Subchronic exposure to ellagic acid impairs cytotoxic T-cell function and suppresses humoral immunity in mice. Immunopharm. Immunot. 25:409–422.
Alm Bergvall, U. and Leimar, O. 2005. Plant secondary compounds and the frequency of food types affect food choice by mammalian herbivores. Ecology 86:2450–2460.
Alm Bergvall, U., Rautio, P., Kesti, K., Tuomi, J., and Leimar, O. 2006. Associational effects of plant defences in relation to within- and between-patch food choice by a mammalian herbivore: neighbor contrast susceptibility and defense. Oecologia 147:253–260.
Amsel, L. P. and Levy, G. 1969. Drug biotransformation interactions in man. II. A pharmacokinetic study of the simultaneous conjugation of benzoic and salicylic acids with glycine. J. Pharm. Sci. 58:321–326.
Aranda, A., Sanchez-Vazquez, F. J., Zamora, S., and Madrid, J. A. 2000. Self-design of fish diets by means of self-feeders: validation of procedures. J. Physiol. Biochem. 56:155–166.
Atsatt, P. R. and O'Dowd, D. J. 1976. Plant defense guilds. Science 193:24–29.
Awaluddin, A. B. and McLean, S. 1985. Conjugation of benzoic acid in marsupials. Aust. J. Zool. 33:693–698.
Baumgart, A., Schmidt, M., Schmitz, H.-J., and Schrenk, D. 2005. Natural furocoumarins as inducers and inhibitors of cytochrome P450 1A1 in rat hepatocytes. Biochem. Pharmacol. 69:657–667.
Behmer, S. T., Simpson, S. J., and Raubenheimer, D. 2002. Herbivore foraging in chemically heterogeneous environments: nutrients and secondary metabolites. Ecology 83:2489–2501.
Boyle, R. and McLean, S. 2004. Constraint of feeding by chronic ingestion of 1,8-cineole in the brushtail possum (Trichosurus vulpecula). J. Chem. Ecol. 30:757–775.
Boyle, R., McLean, S., Foley, W. J., and Davies, N. W. 1999. Comparative metabolism of dietary terpene, p-cymene, in generalist and specialist folivorous marsupials. J. Chem. Ecol. 25:2109–2126.
Boyle, R., McLean, S., Foley, W., Davies, N. W., Peacock, E. J., and Moore, B. 2001. Metabolites of dietary 1,8-cineole in the male koala (Phascolarctos cinereus). Comp. Biochem. Physiol. C 129:385–395.
Brenes-Arguedas, T. and Coley, P. D. 2005. Phenotypic variation and spatial structure of secondary chemistry in a natural population of a tropical tree species. Oikos 108:410–420.
Bridges, J. W., French, M. R., Smith, R. L., and Williams, R. T. 1970. The fate of benzoic acid in various species. Biochem. J. 118:47–51.
Burritt, E. A. and Provenza, F. D. 2000. Role of toxins in intake of varied diets by sheep. J. Chem. Ecol. 26:1991–2005.
Cassini, M. H. 1994. Behavioral mechanisms of selection of diet components and their ecological implications in herbivorous mammals. J. Mammal. 75:733–740.
Clifton, P. G., Burton, M. J., and Sharp, C. 1987. Rapid loss of stimulus-specific satiety after consumption of a second food. Appetite 9:149–156.
Cork, S. J. 1981. Digestion and metabolism in the koala (Phascolarctos cinereus Goldfuss): an arboreal folivore. Ph.D. thesis. University of New South Wales, Australia.
Covelo, F. and Gallardo, A. 2004. Green and senescent leaf phenolics showed spatial autocorrelation in a Quercus robur population in northwestern Spain. Plant Soil 259:267–276.
Dearing, M. D. and Cork, S. 1999. Role of detoxification of plant secondary compounds on diet breadth in a mammalian herbivore, Trichosurus vulpecula. J. Chem. Ecol. 25:1205–1219.
Dearing, M. D. and Schall, J. J. 1992. Testing models of optimal diet assembly by the generalist herbivorous lizard Cnemidophorus murinus. Ecology 73:845–858.
Dearing, M. D., Mangione, A. M., and Karasov, W. H. 2000. Diet breadth of mammalian herbivores: nutrient versus detoxification constraints. Oecologia 123:397–405.
Dearing, M. D., Foley, W. J., and McLean, S. 2005. The influence of plant secondary metabolites on the nutritional ecology of herbivorous terrestrial vertebrates. Annu. Rev. Ecolog. Evol. Syst. 36:169–189.
DeGabriel, J., Foley, W. J., and Wallis, I. R. 2002. The effect of excesses and deficiencies in amino acids on the feeding behavior of the common brushtail possum (Trichosurus vulpecula). J. Comp. Physiol. B 172:607–617.
DiBattista, D. and Sitzer, C. A. 1994. Dietary variety enhances meal size in golden hamsters. Physiol. Behav. 55:381–383.
Double, M. C., Peakall, R., Beck, N. R., and Cockburn, A. 2005. Dispersal, philopatry, and infidelity: dissecting local genetic structure in superb fairy-wrens (Malurus cyaneus). Evolution 59:625–635.
Duncan, A. J., Ginane, C., Gordon, I. J., and Orskov, E. R. 2003. Why do herbivores select mixed diets?, pp. 195–208, in L. t′Mannetje, L. Ramírez-Avilés, C. A. Sandoval-Castro, and J. C. Ku-Vera (eds.). Matching Herbivore Nutrition to Ecosystems Biodiversity. Proceedings of the VI International Symposium on the Nutrition of Herbivores. Universidad Autónoma de Yucatán, Factad de Medicina Veterinaria y Zootecnia, Merida, Mexico.
Foley, W. J., McLean, S., and Cork, S. J. 1995. Consequences of biotransformation of plant secondary metabolites on acid–base metabolism in mammals—a final common pathway. J. Chem. Ecol. 21:721–743.
Foley, W. J., Iason, G. R., and McArthur, C. 1999. Role of plant secondary metabolites in the nutritional ecology of mammalian herbivores: How far have we come in 25 years?, pp. 130–209, in H.-J. G. Jung and G. C. J. Fahey (eds.). Nutritional Ecology of Herbivores. Proceedings of the Vth International Symposium on the Nutrition of Herbivores. American Society of Animal Science, Savoy, USA.
Freeland, W. J. 1991. Plant secondary metabolites: Biochemical coevolution with herbivores, pp. 61–81, in R. T. Palo and C. T. Robbins (eds.). Plant Defenses Against Mammalian Herbivory. CRC Press, Boca Raton, FL, USA.
Freeland, W. J. and Janzen, D. H. 1974. Strategies in herbivory by mammals: the role of plant secondary compounds. Am. Nat. 108:269–289.
Freeland, W. J. and Saladin, L. R. 1989. Choice of mixed diets by herbivores: the idiosyncratic effects of plant secondary compounds. Biochem. Syst. Ecol. 17:493–497.
Freeland, W. J. and Winter, J. W. 1975. Evolutionary consequences of eating: Trichosurus vulpecula (Marsupialia) and the genus Eucalyptus. J. Chem. Ecol. 1:439–455.
Freeland, W. J., Calcott, P. H., and Anderson, L. R. 1985. Tannins and saponin—interaction in herbivore diets. Biochem. Syst. Ecol. 13:189–193.
Galey, F. D., Holstege, D. M., Johnson, B. J., and Siemens, L. 1998. Toxicity and diagnosis of oleander (Nerium oleander) poisoning in livestock, pp. 215–219, in T. Garland and A. C. Barr (eds.). Toxic Plants and Other Natural Toxicants. CABI Publishing, Oxon, UK.
Gardner, C. J., Armour, D. R., Beattie, D. T., Gale, J. D., Hawcock, A. B., Kilpatrick, G. J., Twissell, D. J., and Ward, P. 1996. GR205171: a novel antagonist with high affinity for the tachykinin NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul. Pept. 65:45–53.
Gilardi, J. D., Duffey, S. S., Munn, C. A., and Tell, L. A. 1999. Biochemical functions of geophagy in parrots: detoxification of dietary toxins and cytoprotective effects. J. Chem. Ecol. 25:897–922.
Ginane, C., Baumont, R., Lassalas, J., and Petit, M. 2002. Feeding behavior and intake of heifers fed on hays of various quality, offered alone or in a choice situation. Anim. Res. 51:177–188.
Gous, R. M. and Swatson, H. K. 2000. Mixture experiments: a severe test of the ability of a broiler chicken to make the right choice. Br. Poult. Sci. 41:136–140.
Gregus, Z., Fekete, T., Varga, F., and Klaassen, C. D. 1993. Dependence of glycine conjugation on availability of glycine—role of the glycine cleavage system. Xenobiotica 23:141–153.
Gregus, Z., Fekete, T., Halaszi, E., and Klaassen, C. D. 1996. Does hepatic ATP depletion impair glycine conjugation in vivo? Drug Metab. Dispos. 24:1347–1354.
Griffith, W. H. and Lewis, H. B. 1923. Studies in the synthesis of hippuric acid in the animal organism. V. The influence of amino-acids and related substances on the synthesis and rate of elimination of hippuric acid after the administration of benzoate. J. Biol. Chem. 57:1–24.
Hagele, B. F. and Rowell-Rahier, M. 1999. Dietary mixing in three generalist herbivores: nutrient complementation or toxin dilution? Oecologia 119:521–533.
Harrington, R. A., Hamilton, C. W., Brogden, R. N., Linkewich, J. A., Romankiewicz, J. A., and Heel, R. C. 1983. Metoclopramide—an updated review of its pharmacological properties and clinical use. Drugs 25:451–494.
Hjalten, J., Daneall, K., and Lundberg, P. 1993. Herbivore avoidance by association: vole and hare utilization of woody-plants. Oikos 68:125–131.
Iason, G. R. 2005. The role of plant secondary metabolites in mammalian herbivory: ecological perspectives. Proc. Nutr. Soc. 64:1–9.
Iason, G. R. and Murray, A. H. 1996. The energy costs of ingestion of naturally occurring nontannin plant phenolics by sheep. Physiol. Zool. 69:532–546.
Jakubas, W. J. and Mason, J. R. 1991. Role of avian trigeminal sensory system in detecting coniferyl benzoate, a plant allelochemical. J. Chem. Ecol. 17:2213–2221.
Klasing, K. C. and Calvert, C. C. 2000. The care and feeding of an immune system: an analysis of lysine needs, pp. 253–264, in G. E. Lobley, A. White and J. C. MacRae (eds.). Proceedings of the VIII International Symposium on Protein Metabolism and Nutrition. Wageningen University Press, Wageningen, Netherlands.
Lawler, I. R., Foley, W. J., Pass, G. J., and Eschler, B. M. 1998. Administration of a 5HT3 receptor antagonist increases the intake of diets containing Eucalyptus secondary metabolites by marsupials. J. Comp. Physiol. B 168:611–618.
Lawler, I. R., Foley, W. J., and Eschler, B. M. 2000. Foliar concentration of a single toxin creates habitat patchiness for a marsupial folivore. Ecology 81:1327–1338.
Lee, K. P., Cory, J. S., Wilson, K., Raubenheimer, D., and Simpson, S. J. 2006. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. R. Soc. Lond., B Biol. Sci. 273:823–829.
Lin, J. H. and Lu, A. Y. H. 2001. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu. Rev. Pharmacol. Toxicol. 41:535–567.
Lowry, J. B., Sumpter, E. A., McSweeney, C. S., Schlink, A. C., and Bowden, B. 1993. Phenolic acids in the fibre of some tropical grasses; effect on feed quality and their metabolism by sheep. Aust. J. Agric. Resour. 44:1123–1133.
Mangione, A. M., Dearing, M. D., and Karasov, W. H. 2000. Interpopulation differences in tolerance to creosote bush resin in desert woodrats (Neotoma lepida). Ecology 81:2067–2076.
Marsh, K. J., Foley, W. J., Cowling, A., and Wallis, I. R. 2003. Differential susceptibility to Eucalyptus secondary compounds explains feeding by the common ringtail (Pseudocheirus peregrinus) and common brushtail possum (Trichosurus vulpecula). J. Comp. Physiol. B 173:69–78.
Marsh, K. J., Wallis, I. R., and Foley, W. J. 2005. Detoxification rates constrain feeding in common brushtail possums (Trichosurus vulpecula). Ecology 86:2946–2954.
Marsh, K. J., Wallis, I. R., McLean, S., Sorensen, J. S., and Foley, W. J. 2006. Conflicting demands on detoxification pathways influence how brushtail possums choose their diets. Ecology (in press).
McLean, S. and Duncan, A. J. 2006. Pharmacological perspectives on the detoxification of plant secondary metabolites: implications for ingestive behavior of herbivores. J. Chem. Ecol. (this volume).
McLean, S., Pass, G. J., Foley, W. J., Brandon, S., and Davies, N. W. 2001. Does excretion of secondary metabolites always involve a measurable metabolic cost? Fate of plant antifeedant salicin in common brushtail possum, Trichosurus vulpecula. J. Chem. Ecol. 27:1077–1089.
McLean, S., Brandon, S., Davies, N. W., Foley, W. J., and Muller, H. K. 2004. Jensenone: biological reactivity of a marsupial antifeedant from Eucalyptus. J. Chem. Ecol. 30:19–36.
Milchunas, D. G. and Noy-Meir, I. 2002. Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99:113–130.
Miura, K. and Ohsaki, N. 2004. Diet mixing and its effect on polyphagous grasshopper nymphs. Ecol. Res. 19:269–274.
Moore, B. D., Wallis, I. R., Palá-Paúl, J., Brophy J. J., Willis R. H., and Foley W. J. 2004. Antiherbivore chemistry of Eucalyptus—cues and deterrents for marsupial folivores. J. Chem. Ecol. 30:1743–1769.
Moore B. D., Foley W. J., Wallis I. R., Cowling A., and Handasyde K. A. 2005. Eucalyptus foliar chemistry explains selective feeding by koalas. Biol. Lett. 1:64–67.
Mulder G. J. 1995. Polymorphism in drug conjugation in man: is it a factor of concern in drug toxicity or efficacy? Eur. J. Pharm. Sci. 3:57–59.
Norberg A., Jones W. A., Hahn R. G., and Gabrielsson J. L. 2003. Role of variability in explaining ethanol pharmacokinetics—research and forensic applications. Clin. Pharmacokinet. 42:1–31.
Osawa R., Bird P. S., Harbrow D. J., Ogimoto K., and Seymour G. J. 1993. Microbiological studies of the intestinal microflora of the koala, Phascolarctos cinereus. 1. Colonization of the cecal wall by tannin–protein complex degrading enterobacteria. Aust. J. Zool. 41:599–609.
Pass G. J. and Foley W. J. 2000. Plant secondary metabolites as mammalian feeding deterrents: separating the effects of the taste of salicin from its post-ingestive consequences in the common brushtail possum (Trichosurus vulpecula). J. Comp. Physiol. B 170:185–192.
Pass G. J. and McLean S. 2002. Inhibition of the microsomal metabolism of 1,8-cineole in the common brushtail possum (Trichosurus vulpecula) by terpenes and other chemicals. Xenobiotica 32:1109–1126.
Pass G. J., McLean S., and Stupans I. 1999. Induction of xenobiotic metabolising enzymes in the common brushtail possum, Trichosurus vulpecula, by Eucalyptus terpenes. Comp. Biochem. Physiol. C 124:239–246.
Pfister J. A., Provenza F. D., Manners G. D., Gardner D. R., and Ralphs M. H. 1997. Tall larkspur ingestion: Can cattle regulate intake below toxic levels? J. Chem. Ecol. 23:759–777.
Pfister J. A., Panter K. E., Gardner D. R., Stegelmeier B. L., Ralphs M. H., Molyneux R. J., and Lee S. T. 2001. Alkaloids as anti-quality factors in plants on western US rangelands. J. Range Manag. 54:447–461.
Provenza F. D. 1995. Postingestive feedback as an elementary determinant of food preference and intake in ruminants. J. Range Manag. 48:2–17.
Provenza F. D. 1996. Acquired aversions as the basis for varied diets of ruminants foraging on rangelands. J. Anim. Sci. 74:2010–2020.
Provenza F. D., Pfister J. A., and Cheny C. D. 1992. Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores. J. Range Manag. 45:36–45.
Provenza F. D., Villalba J. J., Cheney C. D., and Werner S. J. 1998. Self-organization of foraging behavior: from simplicity to complexity without goals. Nutr. Res. Rev. 11:199–222.
Radominska-Pandaya A., Czernik P. J., and Little, J. M. 1999. Structural and functional studies of UDP-glucuronosyltransferases. Drug Metab. Rev. 31:817–899.
Rhodes, V. A. and McDaniel, R. W. 2001. Nausea, vomiting, and retching: Complex problems in palliative care. CA Cancer J. Clin. 51:232–248.
Robbins, C. T., Hagerman, A. E., Austin, P. J., McArthur, C., and Hanley, T. A. 1991. Variation in mammalian physiological responses to a condensed tannin and its ecological implications. J. Mammal. 72:480–486.
Rogosic, J., Estell, R. E., Skobic, D., Martinovic, A., and Maric, S. 2006. Role of species diversity and secondary compound complementarity on diet selection of Mediterranean shrubs by goats. J. Chem. Ecol. (this volume).
Rolls, B. J., Rowe, E. A., Rolls, E. T., Kingston, B., Megson, A., and Gunary, R. 1981. Variety in a meal enhances food intake in man. Physiol. Behav. 26:215–221.
Rousset, O. and Lepart, J. 2003. Neighbourhood effects on the risk of an unpalatable plant being grazed. Plant Ecol. 165:197–206.
Scott, L. L. and Provenza, F. D. 2000. Lambs fed protein or energy imbalanced diets forage in locations and on foods that rectify imbalances. Appl. Anim. Behav. Sci. 68:293–305.
Shariatmadari, F. and Forbes, J. M. 1993. Growth and food intake responses to diets of different protein contents and a choice between diets containing two concentrations of protein in broiler and layer strains of chicken. Br. Poult. Sci. 34:959–970.
Singer, M. S., Bernays, E. A., and Carriere, Y. 2002. The interplay between nutrient balancing and toxin dilution in foraging by a generalist insect herbivore. Anim. Behav. 64:629–643.
Singleton, V. L. and Kratzner, F. H. 1969. Toxicity and related physiological activity of phenolic substances of plant origin. J. Agric. Food Chem. 17:497–512.
Snyder, M. J. and Glendinning, J. I. 1996. Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. J. Comp. Physiol. A 179:255–261.
Sorensen, J. S. and Dearing, M. D. 2003. Elimination of plant toxins by herbivorous woodrats: revisiting an explanation for dietary specialization in mammalian herbivores. Oecologia 134:88–94.
Sorensen, J. S., McLister, J. D., and Dearing, M. D. 2005. Plant secondary metabolites compromise the energy budgets of specialist and generalist mammalian herbivores. Ecology 86:125–139.
Stapley, J., Foley, W. J., Cunningham, R., and Eschler, B. 2000. How well can common brushtail possums regulate their intake of Eucalyptus toxins? J. Comp. Physiol. B 170:211–218.
Takeda, N., Morita, M., Hasegawa, S., Horii, A., Kubo, T., and Matsunaga, T. 1993. Neuropharmacology of motion sickness and Emesis—a review. Acta Oto-laryngol. 10–15.
Tamási, V., Vereczkey, L., Falus, A., and Monostory, K. 2003. Some aspects of interindividual variations in the metabolism of xenobiotics. Inflamm. Res. 52:322–333.
Thomas, D. W., Samson, C., and Bergeron, J. 1988. Metabolic costs associated with the ingestion of plant phenolics by Microtus pennsylvanicus. J. Mammal. 69:512–515.
Treit, D., Spetch, M. L., and Deutsch, J. A. 1983. Variety in the flavor of food enhances eating in the rat: a controlled demonstration. Physiol. Behav. 30:207–211.
Tuomi, J. and Augner, M. 1993. Synergistic selection of unpalatability in plants. Evolution 47:668–672.
Villalba, J. J. and Provenza, F. D. 2005. Foraging in chemically diverse environments: energy, protein, and alternative foods influence ingestion of plant secondary metabolites by lambs. J. Chem. Ecol. 31:123–138.
Wallis, I. R., Watson, M. L., and Foley, W. J. 2002. Secondary metabolites in Eucalyptus melliodora: field distribution and laboratory feeding choices by a generalist herbivore, the common brushtail possum. Aust. J. Zool. 50:1–13.
Walton, K., Dorne, J. L., and Renwick, A. G. 2001. Uncertainty factors for chemical risk assessment: interspecies differences in glucuronidation. Food Chem. Toxicol. 39:1175–1190.
Wang, J. and Provenza, F. D. 1997. Dynamics of preference by sheep offered foods varying in flavours, nutrients and a toxin. J. Chem. Ecol. 23:275–288.
Waterlow, J. C. 2006. Protein Turnover. CABI Publishing, Wallingford, UK.
White, R. G. and Lawler, J. P. 2002. Can methane suppression during digestion of woody and leafy browse compensate for energy costs of detoxification of plant secondary compounds? A test with muskoxen fed willows and birch. Comp. Biochem. Phys. A 133:849–859.
Wiggins, N. L., McArthur, C., McLean, S., and Boyle, R. 2003. Effects of two plant secondary metabolites, cineole and gallic acid, on nightly feeding patterns of the common brushtail possum. J. Chem. Ecol. 29:1447–1464.
Wiggins, N. L., McArthur, C., and Davies, N. W. 2006. Diet switching in a generalist mammalian folivore: fundamental to maximising intake. Oecologia 147:650–657.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marsh, K.J., Wallis, I.R., Andrew, R.L. et al. The Detoxification Limitation Hypothesis: Where Did it Come From and Where is it Going?. J Chem Ecol 32, 1247–1266 (2006). https://doi.org/10.1007/s10886-006-9082-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9082-3