Abstract
A number of technologies are available for minimally-invasive cardiac output measurement in patients during surgery but remain little used. A system has been developed based on CO2 elimination (VCO2) by the lungs for use in ventilated patients, which can be fully integrated into a modern anesthesia/monitoring platform, and provides semi-automated, continuous breath-by-breath cardiac output monitoring. A prototype measurement system was constructed to measure VCO2 and end-tidal CO2 concentration with each breath. A baseline measurement of non-shunt cardiac output was made during a brief change in ventilator rate, according to the differential CO2 Fick approach. Continuous breath-by-breath monitoring of cardiac output was then performed from measurement of VCO2, using a derivation of the Fick equation applied to pulmonary CO2 elimination. Automated recalibration was done periodically and data was processed and cardiac output displayed in real time. Measurements were compared with simultaneous measurements by bolus thermodilution in 77 patients undergoing cardiac surgery or liver transplantation. Overall mean bias [sd] for agreement in cardiac output measurement was −0.1 [1.2] L/min, percentage error +44.2%, r = 0.92. Concordance in measurement of changes of at least 15% in cardiac output was 80%. The method followed sudden changes in cardiac output due to arrythmias and run onto cardiopulmonary bypass in real time. The accuracy and precision were comparable to other clinical techniques. The method is relatively seamless and largely automated and has potential for continuous, cardiac output monitoring in ventilated patients during anesthesia and critical care.
Similar content being viewed by others
1 Introduction
Better hemodynamic management is being recognized as important to achieve improvements in patient outcomes during major surgery [1–5]. Minimally-invasive measurement of stroke volume or cardiac output gives vital information required for this, providing data to guide fluid resuscitation and rational use of vasoactive drugs [6].
Various technologies are now available to measure cardiac output in the peri-operative setting. These include technologies based on the differential Fick method applied to carbon dioxide (CO2) elimination by the lungs [7, 8]. The partial CO2 rebreathing method uses this principle to determine non-shunt pulmonary capillary blood flow (\( \dot{Q}c \)). After making baseline measurements of CO2 elimination by the lungs (\( \dot{V}_{{{\text{CO}}_{2} }} \)) and alveolar (end-tidal) CO2 partial pressure (\( PA_{{{\text{CO}}_{2} }} \)), a brief change in alveolar ventilation is made and a repeated measurement of these variables is then done prior to systemic recirculation time, when mixed venous CO2 partial pressure starts to change [9]. These paired sets of measurements allow calculation of \( \dot{Q}c \) on the assumption that \( \dot{Q}c \) and mixed venous CO2 content remain stable. This is the basis of the NICO™ (Respironics Inc. Murrysville PA), which uses an automated partial rebreathing valve and loop proximal to the endotracheal tube to make the change alveolar ventilation by altering serial dead space in the breathing system. This and other available technologies such as arterial pressure-derived cardiac output devices, esophageal Doppler and trans-thoracic bioimpedance devices, have still not achieved widespread use in routine practice, despite the potential for improvements in patient care [10]. This is partly due to the cost of these stand-alone devices and their expensive or cumbersome disposables, and the complexity these devices add to the conduct of anesthesia.
The first study to employ the differential CO2 Fick approach was described by Gedeon et al., and simply employed a change in respiratory rate delivered by the ventilator (accompanied by a change in I:E ratio so that effectively only the end-expiratory pause was altered) to produce the required change in alveolar ventilation [11]. We have shown that the accuracy and precision of cardiac output measurement by a simplified modification of this approach is similar in patients undergoing cardiac surgery to that achievable by the NICO™ and other techniques, and can be made fully automated and hands-free [12–14]. Building on this, a method has been developed based on CO2 elimination by the lungs, which could be fully integrated into modern anesthesia/monitoring platforms using components already existing on these systems, and which provides continuous breath-by-breath cardiac output monitoring in ventilated patients in minimally invasive fashion. A prototype of this system, which has been labeled the “capnotracking” system, was tested by comparison with thermodilution and transesophageal echocardiography in patients undergoing major surgery.
2 Materials and methods
2.1 Measurement system
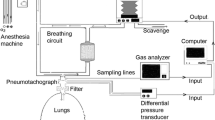
Figure 1 shows a schematic diagram of the system, which consisted of a Datex Capnomac Ultima infrared gas analyzer (Datex-Ohmeda, Helsinki, Finland), and a Fleisch pneumotachograph (Hans Rudolph Inc, Kansas City KS) with inbuilt heating element to prevent water condensation, placed proximal to the hydrophobic breathing circuit filter and attached to a differential pressure transducer (Validyne Corp, Northbridge CA) for measurement of tidal gas flow. Analog data from each of these devices was downloaded continuously at a sampling rate of 200 Hz via a USB analog–digital converter card (USB 6009, National Instruments, Austin, TX), to a notebook computer (MacBook Pro, Apple Corp. Cupertino, CA) for computation. The software system for data download and computation, and instrument control, was constructed using LabView 7.01 (National Instruments, Austin, TX).
\( \dot{V}_{{{\text{CO}}_{2} }} \) and \( PA_{{{\text{CO}}_{2} }} \) were measured with each breath in real time using a capnograph obtained from sidestream gas sampling. \( PA_{{{\text{CO}}_{2} }} \) was taken from an interval on the same early part of the phase 3 plateau of the capnogram both before and during the ventilatory change (to avoid differences in the end-tidal partial pressure with different breath durations that would be expected where the end-tidal plateau was not perfectly flat). \( \dot{V}_{{{\text{CO}}_{2} }} \) for each breath i prior to the ventilatory change (\( \dot{V}_{{{\text{CO}}_{2} i}} \)) was measured by multiplication and integration of inspired and expired flow and CO2 concentration waveforms with respect to time, with appropriate compensation in software for sidestream sampling transport time delay and for the response time of the gas analyzer, and corrected to body temperature and pressure, saturated (BTPS) [15]. The accuracy of the system in measurement of expired tidal volume (VT) integrated from gas flows was verified to within 1% by comparison against a 1 L dry gas syringe across a range of flow rates, incorporating additional calibration to compensate for the effect of static pressure within the breathing system, as well as variations in viscosity of the sampled gas mixture during tidal variations in gas composition. \( \dot{V}_{{{\text{CO}}_{2} }} \) measurement was subsequently calibrated against a volumetrically measured flow of pure CO2 gas fed into a tidally ventilated gas exchange simulator (TTL type 1,600 hinged bellows test lung, Michigan Instruments, Grand Rapids, MI). The coefficient of variation of measured \( \dot{V}_{{{\text{CO}}_{2} }} \) from this simulator was found to be 3% of the target value within the range of ventilator rate settings employed, and that of the \( PA_{{{\text{CO}}_{2} }} \) was 0.25%.
2.2 Clinical protocol
With approval by the local institutional human research ethics committee (Austin Health HREC Project No. 03432, 11/1/2008), adult patients scheduled for either elective cardiac surgery, orthotopic liver transplantation or other major surgery for critical illness requiring invasive monitoring with a pulmonary artery catheter were approached and informed consent obtained for recruitment to the study. Patients were excluded who had a clinical history of severely impaired lung function (FEV1 or FVC <30% of normal predicted). Routine anesthetic management and monitoring was undertaken including insertion of an arterial line and pulmonary artery catheter (Edwards Lifesciences, Irvine, CA) and trans-esophageal echocardiography probe for patients undergoing cardiac surgery. After relaxant general anesthesia was induced and following endotracheal intubation, anesthesia was maintained by either intravenous propofol or inspired isoflurane or sevoflurane, titrated according to clinical indication and bi-spectral index, and a neuromuscular blocker. The endotracheal tube was attached via a breathing filter to a circle absorber system attached to an Aestiva anesthetic machine (Datex-Ohmeda, GE Healthcare, Madison WI). The patient was ventilated with oxygen/air at a minute ventilation adjusted to achieve an end-tidal CO2 partial pressure of approximately 25–35 mmHg. After several minutes, when the patient’s blood pressure and ventilatory status had been stabilized, the gas exchange measurement system (pneumotachograph and gas sampling port) was attached to breathing system just proximal to the breathing filter, and the measurement process was commenced.
Data was collected for a minimum of 30 min or until run onto cardio-pulmonary bypass (CPB) in patients undergoing on-pump cardiac surgery. In this group, where logistic and time constraints permitted, the measurement process was recommenced after separation from CPB and re-establishment of circulation and ventilation of the lungs. Data was again collected for a minimum of 30 min or until separation from the ventilator for transfer to the intensive care unit.
2.3 Measurement algorithm
The technique involves a two-step process:
-
1.
obtaining a baseline measurement of cardiac output (“calibration”) from the differential CO2 Fick method, using a change in alveolar ventilation, and then
-
2.
providing ongoing breath-by-breath monitoring of changes in cardiac output from measured changes in \( \dot{V}_{{{\text{CO}}_{2} }} \) using a relationship derived from consideration of the Fick equation outlined in Appendix 2
-
1.
Baseline cardiac output measurement
The change in alveolar ventilation for baseline cardiac output measurement was made when the standard error of the measured baseline \( \dot{V}_{{{\text{CO}}_{2} }} \) (\( \dot{V}_{{{\text{CO}}_{2} i}} \)) was less than 1.5% of the average value and that of the baseline \( PA_{{{\text{CO}}_{2} }} \) (\( PA_{{{\text{CO}}_{2} i}} \)) was less than 0.1 mmHg for a minimum of 5 consecutive breaths. This was generally achieved within a few minutes of commencement of the measurement process. The ventilatory change involved a step reduction in respiratory rate from a baseline setting of 14 to 6 breaths/min for approximately 45 s. This change was accompanied by a change in I:E ratio from 1:2 to 1:6 so that duration of inspiration, passive expiration and inspiratory pressures remained largely unchanged, in effect only the duration of the end-expiratory pause being altered, consistent with the method proposed by Gedeon et al. [11].
Non-shunt pulmonary capillary blood flow (\( \dot{Q}c \)) was then calculated, as previously described by us [12], using a modification of the differential Fick equation which assumes that the change induced in alveolar ventilation is solely dependent on the change made in respiratory rate (RR). This is the calibration equation:
where \( PB \) is the measured barometric pressure and \( S_{{{\text{CO}}_{2} }} \)was the solubility coefficient of CO2 in blood. The derivation of Eq. 1 is outlined in the Appendix 1. This has been found in our series to significantly improve the precision of measurement of \( \dot{Q}c \), because precision of measurement of \( PA_{{{\text{CO}}_{2} }} \) (from an end-tidal partial pressure) with each breath is generally better than that of \( \dot{V}_{{{\text{CO}}_{2} }} \), particularly during the ventilatory change. \( S_{{{\text{CO}}_{2} }} \)was obtained from the equations of Kelman [16, 17] using a previously published algorithm employing a measured hemoglobin, body temperature, arterial oxygen saturation and base excess obtained from an arterial blood gas sample at the time of measurement, processed on a Radiometer ABL 625 blood gas analyser (Radiometer, Odense, Denmark) [18]. \( PA_{{{\text{CO}}_{2} }} \) during the ventilatory change (\( PA_{{{\text{CO}}_{2} j}} \)) was averaged from the last 4 breaths of the period of change.
\( \dot{Q}c_{i} \) was then corrected using a non-invasive estimate of pulmonary shunt fraction (\( {{\dot{Q}s} \mathord{\left/ {\vphantom {{\dot{Q}s} {\dot{Q}t}}} \right. \kern-\nulldelimiterspace} {\dot{Q}t}} \)) obtained from a pulse oximetry saturation measurement (\( Sp_{{{\text{O}}_{2} }} \)) and an assumed mixed venous saturation (\( S\bar{v}_{{{\text{O}}_{2} }} \)) of 70%, according to the method of Rasanen et al. [19, 20]
Finally, to obtain total pulmonary blood flow (cardiac output) \( \dot{Q}t \):
This process was repeated once over the space of approximately 5 min at the commencement of measurement and the results averaged to give baseline \( \dot{Q}t \)(\( \dot{Q}t_{i} \)).
-
2.
Ongoing breath-by-breath cardiac output monitoring
Continuous ongoing breath-by-breath measurement of \( \dot{Q}t \) at any subsequent breath k was then made using the continuity equation, derivation of which is outlined in the Appendix 2 and Fig. 7.
Because changes in alveolar ventilation will alter \( \dot{V}_{{{\text{CO}}_{2} k}} \) irrespective of the cardiac output, where the minute ventilation was altered by the anesthesiologist the measured value of \( \dot{V}_{{{\text{CO}}_{2} k}} \) was simply scaled according to the change in alveolar minute ventilation (calculated using an estimate of anatomical deadspace based on height and weight) relative to the baseline value until a further recalibration measurement was made.
Because pulmonary \( \dot{V}_{{{\text{CO}}_{2} }} \) is ultimately dictated by metabolic CO2 production, which is buffered in the short term by substantial body CO2 stores, \( \dot{V}_{{{\text{CO}}_{2} k}} \) following a sustained change in cardiac output will normalise toward baseline. Therefore this baseline “calibration” measurement was repeated (“recalibration”) every 30 min during the procedure.
A six breath moving average was used to smooth the resulting value for \( \dot{Q}t_{k} \). At intervals of approximately 10 min during this ongoing measurement of \( \dot{Q}t_{k} \) a measurement was simultaneously made by bolus thermodilution (\( \dot{Q}Td \)) using an average of 3 boluses with room temperature saline. The boluses were not timed with respiration, and to minimise bias, given that the measurements were not blinded to each other, all thermodilution measurements were averaged, only excluding outliers more than 3 standard deviations from the mean of the other two and repeating the excluded measurement (to deal with occasional artefactual measurements).
In addition, in a subset of 10 patients undergoing cardiac surgery, measurements of \( \dot{Q}t_{k} \) were made during staged run onto cardio-pulmonary bypass (CPB), by increasing venous drainage into the reservoir of the bypass pump and pump flow, moving from partial to full CPB. This permitted pulmonary blood flow to be progressively reduced in controlled stepwise fashion and held steady for 1 min while measurement data was collected. Since thermodilution is not a suitable technique for cardiac output measurement during this process (due to loss of part of the thermal bolus into the CPB reservoir), the change in the reference cardiac output was calculated from changes in heart rate and in velocity–time integral (VTI) measured in the left ventricular outflow tract by pulse wave Doppler using the trans-esophageal echocardiography probe.
2.4 Statistical analysis
Overall agreement between \( \dot{Q}t_{k} \) and \( \dot{Q}Td \) was determined by calculation of mean difference (bias) and standard deviation of the difference (precision) between paired measurements made by the two techniques, with adjustment for multiple measurements per patient as described by Bland and Altman [21, 22], and by calculation of the intraclass correlation coefficient r [23].
To assess the ability of the method to follow changes in cardiac output, concordance in the measurement of the direction of change from the previous measurement of both \( \dot{Q}t_{k} \) and \( \dot{Q}Td \) in all patients was calculated after the method of Perrino et al., with an exclusion zone of the central ±15% of measurements as recommended by Critchley et al. [24, 25].
3 Results
Seventy-seven patients (56 male and 21 female) were recruited to the study, of whom forty-eight underwent cardiac surgery, twenty-eight underwent liver transplantation, and one general surgery for abdominal sepsis. Data was collected in the operating room in seventy-two patients and in the intensive care unit in five patients. Mean age was 58.0 years (range 19–85 years) and mean weight was 82.8 kg (range 52–117 kg). A total of 452 bolus thermodilution measurements were made with a median of 4 (range 1–10) measurements per patient. The mean \( \dot{Q}Td \) was 5.4 L/min (range 0.7–16.5 L/min). The minimum time to the first calibration cardiac output measurement was 4 min and the median time was 5½ min (interquartile range 4.8–7.5 min, with a 95th centile of 9.9 min), following commencement of the measurement process. A median of 2 calibration measurements (range 1–4) were made per patient during the data collection period. The mean duration of data collection was 47 min (standard deviation of 23 min, range 9–125 min). The wide range mainly reflects the differences in available time between patients having cardiac valve surgery and those having coronary artery surgery requiring multiple graft harvesting, prior to run onto cardiopulmonary bypass.
3.1 Accuracy and precision
The mean bias between the methods (\( \dot{Q}t_{k} - \dot{Q}Td \)) was −0.1 L/min [95% confidence limits: ±0.1 L/min], with a standard deviation of the difference between paired measurements of 1.2 L/min, giving limits of agreement between the two methods of +2.3 and −2.5 L/min (percentage error of 44.2%). Figure 2 is a Bland and Altman plot showing difference versus average of the two methods and Fig. 3 shows the correlation between them. The intraclass correlation coefficient was r = 0.92. The line of best fit followed the relationship \( \dot{Q}t_{k} = (0. 90.\dot{Q}Td + 0. 4 1) \) L/min. Agreement between the two methods for the first measurement only in each patient was a mean bias of −0.2 L/min, with a standard deviation of the difference of 1.3 L/min and percentage error of 46.3%, r = 0.93.
3.2 Trending
Figure 4 shows a four-quadrant plot of the relative change from the previous measurement in cardiac output measured by both techniques. Concordance between the system being tested and thermodilution was 80%.
Data collected in the subgroup of 10 patients undergoing cardiac surgery during staged run onto CPB is shown in Fig. 5a, b. Figure 5a shows a typical pattern of acute change in cardiac output measured by the system and the reference method during this process. Figure 5b shows correlation of measured percentage change in cardiac output by the system and the reference method (trans-esophageal Doppler) in all 10 patients in this subgroup. The slope of this relationship was \( d\dot{Q}t_{k} = (0. 8 3.d\dot{Q}_{Td} + 1 1) \) %, r = 0.87. This indicated that the continuity equation (Eq. 4) effectively characterizes the relationship between acute changes in pulmonary blood flow and CO2 elimination by the lungs.
Data illustrating the measurement of cardiac output during staged run onto cardiopulmonary bypass (CPB) in a subset of 10 patients. a Shows typical data from a patient, where reference measurements were made by bolus thermodilution during coronary artery graft harvesting, and using Doppler measurements during run onto CPB. b Shows the relationship between change in cardiac output measured by the system being tested (\( d\dot{Q}t_{k} \)) and the Doppler-based reference method (\( d\dot{Q}_{Doppler} \)) in all 10 patients
Figure 6a shows incidental data collected from one patient where a sudden unexpected ventricular tachyarrythmia caused cardiac arrest during coronary artery graft harvesting. This event was captured in real time by the system being tested, along with subsequent measurements made during attempted resuscitation and urgent cannulation for CPB. Figure 6b shows data collected from another patient during coronary artery graft harvesting who had two episodes of atrial fibrillation treated with electrical cardioversion (DCR). The changes in cardiac output measured by the capnotracking system and thermodilution are shown.
Incidental data from patients undergoing cardiac surgery showing measurement of acute changes in cardiac output by the method being tested prior to and during run onto cardiopulmonary bypass (CPB). a Sudden ventricular tachycardia (VT) with haemodynamic collapse, followed by urgent cannulation for cardio-pulmonary bypass (CPB). b Two episodes of rapid atrial fibrillation with acute falls in cardiac output, each treated successfully with direct current reversion (DCR)
4 Discussion
The current study demonstrates that continuous breath-by-breath monitoring of cardiac output is achievable in ventilated patients using a system based on CO2 elimination by the lungs, and can deliver accurate tracking of pulmonary blood flow during unstable hemodynamics.
This is an attractive approach as it presents a seamless means for cardiac output monitoring which needs no devices additional to those already integral to a modern anesthesia/monitoring platform, and can be made largely automated and hands-free. This is particularly the case now when modern electronic ventilators which allow automated manipulation in software of ventilatory settings, including rate, volume and I:E ratio, are standard equipment for both anesthesia and critical care. These ventilators are fully integrated on many currently available modern anesthesia platforms, and capable of automated control using an algorithm similar to that employed in the current study. In this way it has advantages over other techniques employing CO2 elimination by the lungs, such as the partial CO2 rebreathing method, which requires the addition of a rebreathing loop to the breathing system [8], and other approaches that have been described which have measured mixed venous gas content directly using invasive catheterisation to calculate pulmonary blood flow using the Fick equation [26, 27].
Our previously published study in patients undergoing cardiac surgery showed that replication of the original method of Gedeon et al. [11], using a change in end-expiratory pause alone, could result in accuracy and precision of measurement of cardiac output comparable to other technologies, in patients undergoing cardiac surgery [12–14]. However, this method suffered the same limitations as the partial CO2 rebreathing method, requiring relatively stable hemodynamics during the measurement process for accuracy. The current study then extends the capability of this approach, and shows that CO2 elimination by the lungs can be used to successfully track substantial changes in pulmonary blood flow in real time following this baseline measurement, using the relationship described by Eq. 4. This study validates this in a wider population of patients, and a much wider range of cardiac output by inclusion of patients with liver failure and hyperdynamic circulation.
A larger change in alveolar ventilation was used than in our previous study, which would be expected to improve the precision of measurement of the baseline cardiac output. Maximal accuracy and precision of the calibration cardiac output measurement will be obtained where the largest possible change in breath rate is made in the calibration maneuver. However, this needs to be within limits that avoid unphysiological ventilation that might be harmful or alter respiratory mechanics on the one hand, and allow the maneuver to be completed within systemic recirculation time on the other. In common with the partial CO2 rebreathing method, recirculation of mixed venous blood will lead to underestimation of high cardiac outputs [9]. The duration of the calibration maneuver in this study was similar to that employed by the partial CO2 rebreathing method, and there was no evidence from Figs. 2 or 3 that this error occurred in this series. In our experience an approximately two-fold change in breath rate (i.e., either halved or doubled) is the ideal change to be made for the calibration maneuver, and represents no significant risk to patients over the duration of the maneuver.
The overall precision in agreement with thermodilution found in the study is similar to that found in a recently published meta-analysis of the accuracy and precision of four minimally invasive approaches to peri-operative cardiac output measurement [14]. The validity of the continuity equation for tracking of changes in cardiac output is shown by the data in Fig. 4 for concordance between the method and thermodilution. This is reinforced by the data from the subset of patients during staged run onto CPB (Fig. 5b), which tested the real-time response of the method to sudden dramatic falls in pulmonary blood flow. The response time of the system to sudden changes is increased by smoothing of the \( \dot{V}_{{{\text{CO}}_{2} }} \) signal, but is similar to that seen in pulse oximeters under clinical conditions. This data, and those in Fig. 6a, b captured during sudden arrythmias in patients, suggests that hemodynamically induced change in \( \dot{V}_{{{\text{CO}}_{2} }} \) provides robust real time monitoring of critical changes in cardiac output, provided that ventilation of the lungs continues during this period. This supports the suggestions made by a number of authors in the past that CO2 elimination, in the form of an end-tidal concentration measurement, is useful in guiding cardiopulmonary resuscitation [28–30]. Published case reports show that many available methods, including thermodilution, do not deliver reliable measurements during such critical incidents [31, 32]. With this in mind the data captured in Fig. 6b is encouraging.
Other authors have suggested that a useful relationship can be drawn between change in pulmonary blood flow and CO2 elimination by the lungs, which might provide a basis for cardiac output measurement. Breen and Isserles, using data from animal and computer models, proposed a linear relationship [33, 34]. The continuity equation used in the current study is not linear, but the change in \( \dot{V}_{{{\text{CO}}_{2} }} \) it predicts approximates their equation within roughly two-fold changes in pulmonary blood flow either side of a given baseline value. Outside of these bounds the two approaches diverge, the linear model producing a somewhat flatter response to larger increases in cardiac output. Nevertheless, their data support the accuracy of the methodology tested in the current study. Both approaches assume that both \( \dot{V}_{{{\text{CO}}_{2} }} \) and the arterio-venous CO2 content difference in pulmonary blood will change in response to changes in pulmonary blood flow. In steady state conditions, \( \dot{V}_{{{\text{CO}}_{2} }} \) is ultimately dictated by metabolic production of CO2. However, substantial buffering of CO2 content by body tissue stores means that CO2 elimination by the lungs is slow to reflect changes in metabolic production of CO2, but more immediately reflects changes in pulmonary blood flow [34]. The data from the current study support this assumption during the interval between recalibrations. Animal data suggests that the rate of recovery of pulmonary \( \dot{V}_{{{\text{CO}}_{2} }} \) following a sustained change in cardiac output is slow enough to justify a 30 min recalibration interval [33]. A shorter interval may produce improved accuracy but would cause more frequent interruption to continuous cardiac output monitoring.
The method has a number of limitations in comparison with other techniques currently available for minimally-invasive cardiac output monitoring. It is adapted only to patients undergoing controlled ventilation, and so its use is restricted to the intra-operative and critical care environments. Some delay in collection of the baseline cardiac output measurement may accompany unstable gas exchange and hemodynamics, for instance immediately following weaning from cardiopulmonary bypass. It is not envisaged that the method would replace the pulmonary artery catheter in high risk cardiac surgery, where measurement of pulmonary artery pressures is often essential in addition to cardiac output measurement.
The methodology applied in this study for generation of the baseline cardiac output is designed to function where controlled mandatory ventilation is applied, not other ventilatory modes such as SIMV or pressure supported spontaneous ventilation, where respiratory rate and pattern are not controlled. The need to correct for subsequent elective changes in minute ventilation by the anesthesiologist, by changes in either tidal volume or rate, is a further limitation. However, practical management of this was found to be achieved as described by a simple correction using a scaling factor based on estimated change in alveolar minute ventilation, followed by an automated recalibration after 5 min during a subsequent stable period. A minimum of 5 min after the change allowed for a new steady state in CO2 elimination to be approached, making a recalibration measurement valid.
Two clinical scenarios invalidate the assumptions inherent in Eq. 4. The first is the sudden release of accumulated CO2 from an ischemic limb after release of a tourniquet or vascular clamp. A transient rise in CO2 elimination will produce an overestimate of cardiac output from Eq. 4 relative to the real changes that may occur due to the sympathetic response to acute hypercapnia and release of metabolites into the circulation. The second scenario is laparoscopic surgery, where CO2 absorption from the peritoneum, which is substantial in some patients, increases body CO2 output, and produces an artefactual rise in calculated pulmonary flow. For this reason it is necessary to recalibrate the system after establishment of CO2 pneumoperitoneum using Eq. 1. An opposite error in calculated pulmonary flow accompanies intra-operative hypothermia which causes a fall in metabolic rate and CO2 production. This is readily corrected in real-time by patient temperature measurement, with correction of measured \( \dot{V}_{{{\text{CO}}_{2} }} \) by 7% for each degree temperature change from that recorded at the time of the baseline calibration [35]. As mentioned above, changes made in alveolar minute ventilation will alter \( \dot{V}_{{{\text{CO}}_{2} k}} \) due to washout or washin of CO2 from the lungs and body tissues. It was found that a simple scaling of the measured \( \dot{V}_{{{\text{CO}}_{2} k}} \) according to the change in alveolar minute ventilation relative to the baseline provided a satisfactory correction for this until an automated recalibration measurement, which was triggered within 5 min of the change.
A changing hemoglobin concentration, due for example to blood loss or hemodilution, affects the solubility of CO2 in blood and will influence the calculated pulmonary flow from Eq. 4. The surface plot shown in Fig. 7 in Appendix 2 is shifted by this in such a fashion that a 20% fall in the hemoglobin concentration will cause the cardiac output to be underestimated by approximately 10%. This is corrected by manually updating the hemoglobin concentration within the program where required. New generation pulse oximetry devices which also allow the non-invasive monitoring of hemoglobin concentration will allow this to also become automated in the future.
Graphical representation using a 3-dimensional surface plot of the relationships between the three physiological variables of the Fick equation: \( \dot{V}_{{{\text{CO}}_{2} }} \), the net rate of elimination of CO2 by the lungs; \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \), the arterio-venous content difference of CO2 in blood; and \( \dot{Q}t \), the cardiac output
Techniques based on CO2 elimination await validation in patients with severe lung disease. Anecdotal experience from the current study has suggested that the Gedeon method for obtaining the baseline cardiac output measurement may be inaccurate in patients with severe airflow obstruction with gas trapping, where a prolonged end-expiratory pause during the ventilatory maneuver may actually cause relative improvement in gas exchange out of proportion to the acute fall in \( \dot{V}_{{{\text{CO}}_{2} }} \) expected in patients with normal lungs. In such patients and other patients with severely impaired gas exchange, such as those in critical care with respiratory failure for instance, a method for cardiac output measurement based on lung gas exchange is not the preferred method, and another minimally invasive approach is likely to be more reliable.
In conclusion, a minimally-invasive system based on CO2 elimination by the lungs and the Fick principle delivered continuous real time monitoring of cardiac output in patients during major surgery with accuracy and precision in measurement comparable to other methods. The advantage of this approach is its potential to be fully and seamlessly integrated using appropriate software algorithms into modern anesthesia delivery platforms of the type now widely used for patient care. The method might be a useful and accessible alternative for minimally-invasive cardiac output measurement in perioperative monitoring for ventilated patients.
References
Donati A, Loggi S, Preiser JC, Orsetti G, Munch C, Gabbanelli V, Pelaia P, Pietropaoli P. Goal-directed introperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 2007;132(6):1817–24.
Chytra I, Pradl R, Bosman R, Pelnář P, Kasal E, Židková A. Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: a randomized controlled trial. Crit Care. 2007;11:R24.
Bundgaard-Nielsen M, Holte K, Secher NH, Kehlet H. Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol Scand. 2007;51:331–40.
Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109(4):723–40.
Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia. 2008;63:44–51.
Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887–97.
Blomqvist H, Frostell C, Hedenstierna G. A non-invasive technique for measurement of lung perfusion. Intensive Care Med. 1986;12:172–8.
Capek JM, Roy RJ. Noninvasive measurement of cardiac output during partial CO2 rebreathing. IEEE Trans Biomed Eng. 1988;35:653–61.
Yem JS, Tang Y, Turner MJ, Baker AB. Sources of error in noninvasive pulmonary blood flow measurements by partial rebreathing: a computer model study. Anesthesiology. 2003;98(4):881–7.
Miller TE, Roche AM, Gan TJ. Poor adoption of hemodynamic optimization during major surgery: are we practicing substandard care? Anesth Analg. 2011;112(6):1274–6.
Gedeon A, Forslund L, Hedenstierna G, Romano E. A new method for noninvasive bedside determination of pulmonary blood flow. Med Biol Eng Comput. 1980;18:411–8.
Peyton P, Thompson D, Junor P. Non-invasive automated measurement of cardiac output during stable cardiac surgery using a fully integrated differential CO2 Fick method. J Clin Monit Comput. 2008;22(4):285–92.
Botero M, Kirby D, Lobato E, Staples E, Gravenstein N. Measurement of cardiac output before and after cardiopulmonary bypass: comparison among aortic transit-time ultrasound, thermodilution, and noninvasive partial CO2 rebreathing. J Cardiothorac Vasc Anesth. 2004;18(5):563–72.
Peyton P, Chong S. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology. 2010;113(5):1220–35.
Noguchi H, Ogushi Y, Yoshiya I, Itakura N, Yamabayashi H. Breath-by-breath \( \dot{V}_{{{\text{CO}}_{2} }} \) and \( \dot{V}_{{{\text{O}}_{2} }} \) require compensation for transport delay and dynamic response. J Appl Physiol Respir Environ Exerc Physiol. 1981;51(6):1662–1675.
Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol. 1966;21(4):1375–6.
Kelman GR. Digital computer procedure for the conversion of PCO2 into blood CO2 content. Respir Physiol. 1967;3:111–5.
Peyton P, Robinson G, Thompson B. The effect of ventilation-perfusion inhomogeneity and nitrous oxide on oxygenation in anesthesia: physiological modeling of gas exchange. J Appl Physiol. 2001;91:17–25.
Räsänen J, Downs JB, Hodges MR. Continuous monitoring of gas exchange and oxygen use with dual oximetry. J Clin Anesth. 1988;1(1):3–8.
Cohen JB, Camporesi EM, Schweiger HW. Respiratory monitoring. In: Wilson WC, Grande CM, Hoyt DB, editors. Trauma, critical care. Vol II. Informa healthcare: New York; 2007. Ch 8. p. 150
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–10.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82.
Streiner DL, Norman GR. Health measurement scales. A practical guide to their development and use. 2nd edn. Oxford: Oxford Medical publications; 1995. Ch 8. pp. 104–26.
Perrino AC Jr, Harris SN, Luther MA. Intraoperative determination of cardiac output using multiplane transesophageal echocardiography: a comparison to thermodilution. Anesthesiology. 1998;89:350–7.
Critchley LA, Lee A, Ho AM-H. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111:1180–92.
Mahutte CK, Jaffe MB, Sassoon CS, Wong DH. Cardiac output from carbon dioxide production and arterial and venous oximetry. Crit Care Med. 1991;19(10):1270–7.
Davies GG, Jebson PJ, Glasgow BM, Hess DR. Continuous Fick cardiac output compared to thermodilution cardiac output. Crit Care Med. 1986;14(10):881–5.
Trevino RP, Bisera J, Weil MH, Rackow EC, Grundler WG. End-tidal CO2 as a guide to successful cardiopulmonary resuscitation: a preliminary report. Crit Care Med. 1985;13:910–1.
Garnett A, Ornato JP, Gonzalez ER, Johnson B. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. JAMA. 1987;257:512–5.
Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. NEJM. 1988;318:607–11.
Vannucci A, Krejci V, Kangrga I. Performance of Vigileo and LiDCOplus cardiac output monitors during a prolonged cardiac arrest and resuscitation. Eur J Anaesthesiol. 2009;26:885–7.
Nishiwaki C, Kotake Y, Yamada T, Nagata H, Tagawa M, Takeda J. Response time of different methods of cardiac output monitoring during cardiopulmonary resuscitation and recovery. J Cardiothorac Vasc Anesth. 2010;24(2):306–8.
Isserles SA, Breen PH. Can changes in end-tidal PCO2 measure changes in cardiac output? Anesth Analg. 1991;73:808–14.
Breen PH. How do changes in exhaled CO2 measure changes in cardiac output? A numerical analysis model. J Clin Monit Comput. 2010;24:413–9.
Ganong WF. Review of medical physiology. 16th ed. East Norwalk, CT: Appleton and Lange; 1993. p. 256.
Acknowledgments
Support was kindly provided from a Project Grant from the Australian and New Zealand College of Anaesthetists.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
1.1 Derivation of the calibration equation (measurement of the baseline cardiac output)
According to standard theory of the differential CO2 Fick method [7, 8, 10, 11] non-shunt pulmonary capillary blood flow (\( \dot{Q}c \)) can be calculated non-invasively during a period of hemodynamic stability where alveolar ventilation (\( \dot{V}A \)) is changed. If \( \dot{Q}c \) and mixed venous CO2 blood content are considered constant during the change (prior to systemic recirculation)
where i and j represent, respectively, breaths prior to and during the induced change in \( \dot{V}A \), \( PA_{{{\text{CO}}_{2} }} \) is the alveolar partial pressure of CO2 and \( \dot{V}_{{{\text{CO}}_{2} }} \) is the measured CO2 elimination rate. \( PB \) is the measured barometric pressure and \( S_{{{\text{CO}}_{2} }} \)is the slope of the solubility curve of carbon dioxide in blood at a given partial pressure, obtained from the equations of Kelman [16, 17], using a previously published algorithm employing a measured hemoglobin, body temperature, arterial oxygen saturation and base excess obtained from an arterial blood gas sample at the time of measurement [18].
Using the theory of Gedeon et al. [11], if \( \dot{V}A \) is changed by changing respiratory rate (RR) and I:E ratio such that only the duration of the end-expiratory pause is altered, maintenance of the stability of pulmonary mechanics and deadspace during the measurement maneuver can be assumed. This allows a mathematical simplification to be adopted for the measurement of the numerator of Eq. 5, since the change induced in \( \dot{V}A \) can be assumed to be solely dependent on the change made in respiratory rate (RR) [11]. Thus
where \( \dot{V}Ai \) and \( \dot{V}Aj \) are \( \dot{V}A \) prior to and during the change respectively. \( \dot{V}Ai \) can be estimated from \( \dot{V}_{{{\text{CO}}_{2} i}} \) and \( PA_{{{\text{CO}}_{2} i}} \) according to
and likewise for \( \dot{V}Aj \)
This is substituted in the numerator of Eq. 5 to give Eq. 1. This has been found to significantly improve the precision of measurement of \( \dot{Q}c \), because precision of measurement of \( PA_{{{\text{CO}}_{2} }} \) (from and end-tidal measurement) is generally better than that of \( \dot{V}_{{{\text{CO}}_{2} }} \), particularly during the ventilatory change.
Appendix 2
2.1 Derivation of the continuity equation
The Fick equation, based on the principle of conservation of mass, for CO2, states that
where \( \dot{V}_{{{\text{CO}}_{2} }} \) is the rate of exchange of CO2 by the lungs (a negative number as CO2 is eliminated by the lungs). The content of CO2 in mixed venous blood and arterial blood is \( C\bar{v}_{{{\text{CO}}_{2} }} \) and \( Ca_{{{\text{CO}}_{2} }} \) respectively.
Considering the arterio-venous content difference of CO2 as a single variable (\( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \)):
Equation 11 for two breaths i and k can be combined
and transposed, so that,
While \( \dot{V}_{{{\text{CO}}_{2} }} \) can be measured non-invasively, Eq. 13 has two unknowns, \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \) and \( \dot{Q}t \). The relationship of these three variables can be displayed graphically using a 3-dimensional surface plot, with \( \dot{Q}t \) as the vertical axis, as shown in Fig. 7. The units chosen are typical values over a physiologically relevant range.
For the purposes of the analysis, \( \dot{Q}t \) is the independent variable. Movement in \( \dot{V}_{{{\text{CO}}_{2} }} \) and \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \) in response to a change in \( \dot{Q}t \) follows a path simultaneously described by two unit vectors in the planes \( \dot{V}_{{{\text{CO}}_{2} }} - \dot{Q}t \) and \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} - \dot{Q}t \). These are respectively described by the partial derivatives of \( \dot{V}_{{{\text{CO}}_{2} }} \) and \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \) with respect to \( \dot{Q}t \):
and
From this, the relationships between the changes in \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \) and \( \dot{V}_{{{\text{CO}}_{2} }} \), respectively, in response to a change in \( \dot{Q}t \), are given by
Transposing and integrating both sides gives:
where m and n are respective constants of integration.
For a given breath i and a subsequent breath k:
and
Taking antilogs and transposing yields
This indicates that, in response to a change in \( \dot{Q}t \) occurring, \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \) and \( \dot{V}_{{{\text{CO}}_{2} }} \) will change in proportionally equal opposite directions to each other, according to
Combining Eq. 21 with Eq. 12 gives
Equation 4 allows determination of an accurate estimate of \( \dot{Q}t \) for any breath k following a measurement of \( \dot{Q}t \) and \( \dot{V}_{{{\text{CO}}_{2} }} \) at a previous breath i.
The relationship is illustrated in Fig. 7 for a change in \( \dot{Q}t \) and its predicted effect on \( \dot{V}_{{{\text{CO}}_{2} }} \) and \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \). The path described by this relationship is shown as black dots in Fig. 1, for a scenario in which \( \dot{Q}t \) declines stepwise from 8 to 4, 2, and 1 L/min. At any given point along the line, the slope of the line connecting these points is maximal and is the directional derivative of the multivariate function described by the Fick equation. Assuming no other physiological influences act on either \( \dot{V}_{{{\text{CO}}_{2} }} \) or \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \), the change in each follows this line as \( \dot{Q}t \) changes. The clinical situations which represent common exceptions to this assumption are discussed above.
The arterio-venous content difference \( C\left( {a - \bar{v}} \right)_{{{\text{CO}}_{2} }} \) is expected to more than double during this process. CO2 stores in the body are very large (over 100 L), so that a change in \( \dot{V}_{{{\text{CO}}_{2} }} \) in response to a sustained change in \( \dot{Q}t \) will itself be sustained, maintaining the validity of Eq. 4 as a useful approximation for in excess of half an hour, as confirmed by experimental data [31].
Rights and permissions
About this article
Cite this article
Peyton, P.J. Continuous minimally invasive peri-operative monitoring of cardiac output by pulmonary capnotracking: comparison with thermodilution and transesophageal echocardiography. J Clin Monit Comput 26, 121–132 (2012). https://doi.org/10.1007/s10877-012-9342-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-012-9342-4