Abstract
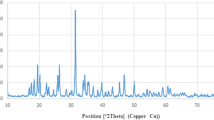
Recently, Simvastatin (SIM) is received notable attention due to its anti-cancer properties. However, the lipophilicity feature of SIM restricted its application as a chemotherapy agent. This work aimed to investigate the Polycaprolactone–Polyethylene Glycol (PCL-PEG) efficiency as the nano-carrier for SIM to enhance its anti-cancer properties. Dynamic Light Scattering (DLS), field emission scanning electron microscopy (FE-SEM), and Fourier transform infrared (FTIR) were used to characterize drug-loaded nanoparticles (NPs). To study apoptotic and anti-proliferative properties of drug-loaded NPs, flow cytometry and MTT assays were utilized. The real-time PCR method measured the expression of apoptotic genes Bcl-2, Bax, Fas and cell cycle regulating genes, p53, cyclin D, and Rb. MTT assay indicated that loading SIM on PCL-PEG NPs increased cytotoxicity dose-dependently. In addition, a noticeable increase was found in the sub- G1 population of treated cells with drug-loaded NPs than free SIM. The remarkable up-regulation of Bax, Fas and p53 genes was detected in treated MCF-7 cells with the decrease of Bcl2, Rb, and Cyclin D 1 gene expression in cells treated with drug-loaded NPs than free SIM. Taken together, these results show that the polymeric PCL-PEG NPs systems represent a promising new therapeutic approach for the treatment of breast cancer.










Similar content being viewed by others
References
K. Nurgali, R. T. Jagoe, and R. Abalo (2018). Adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Fronti. Pharmacol. 9, 245.
Y. Tang, et al. (2016). Classification, treatment strategy, and associated drug resistance in breast cancer. Clinical Breast Cancer 16 (5), 335–343.
E. Miller, et al. (2014). Current treatment of early breast cancer: adjuvant and neoadjuvant therapy. F1000Research 3, 198.
G. Fritz, et al. (2002). Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer 87 (6), 635–644.
K. Sheikholeslami, et al. (2019). Simvastatin induces apoptosis in medulloblastoma brain tumor cells via mevalonate cascade prenylation substrates. Cancers 11 (7), 994.
K. Nejati-Koshki, A. Akbarzadeh, and M. Pourhassan-Moghaddam (2014). Curcumin inhibits leptin gene expression and secretion in breast cancer cells by estrogen receptors. Cancer Cell Internat. 14 (1), 1–7.
S. Samadzadeh, et al. (2021). In vitro anticancer efficacy of Metformin-loaded PLGA nanofibers towards the post-surgical therapy of lung cancer. J. Drug Delivery Sci. Technol. 61, 102318.
P. M. Tricarico, S. Crovella, and F. Celsi (2015). Mevalonate pathway blockade, mitochondrial dysfunction and autophagy: a possible link. Int. J. Mol. Sci. 16 (7), 16067–16084.
M. Thurnher, G. Gruenbacher, and O. Nussbaumer (2013). Regulation of mevalonate metabolism in cancer and immune cells. Biochimica et Biophysica Acta (BBA) Molecular and Cell Biology of Lipids 1831 (6), 1009–1015.
J. Clendening and L. Penn (2012). Targeting tumor cell metabolism with statins. Oncogene 31 (48), 4967–4978.
E. Arabizadeh, et al. (2019). Potential effect of simvastatin as an anti-cancer agent on SOX7 and SOX9 expression in prostate cancer cell lines. Shiraz E-Med J. https://doi.org/10.5812/semj.69057.
A. El-Sohemy and M. C. Archer (2000). Inhibition of N-methyl-N-nitrosourea-and 7, 12-dimethylbenz [a] anthracene-induced rat mammary tumorigenesis by dietary cholesterol is independent of Ha-Ras mutations. Carcinogenesis 21 (4), 827–831.
G. P. Nagaraju, et al. (2012). The impact of curcumin on breast cancer. Integrative Biol. 4 (9), 996–1007.
A. Firouzi-Amandi, et al. (2018). Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed. Pharmacotherapy 105, 773–780.
R. Zamani, et al. (2018). Recent advances in cell electrospining of natural and synthetic nanofibers for regenerative medicine. Drug Res. 68 (08), 425–435.
M. Dadashpour, et al. (2018). Watercress-based electrospun nanofibrous scaffolds enhance proliferation and stemness preservation of human adipose-derived stem cells. Artificial Cells, Nanomed. Biotechnol. 46 (4), 819–830.
K. Nejati, et al. (2021). Biomedical applications of functionalized gold nanoparticles: a review. J Cluster Sci. https://doi.org/10.1007/s10876-020-01955-9.
Y. Panahi, et al. (2017). Preparation, surface properties, and therapeutic applications of gold nanoparticles in biomedicine. Drug Res. 11 (02), 77–87.
M. Zamani, et al. (2018). In vitro and in vivo biocompatibility study of folate-lysine-PEG-PCL as nanocarrier for targeted breast cancer drug delivery. European Polymer J. 103, 260–270.
H. Nosrati, et al. (2019). Biotin-functionalized copolymeric PEG-PCL micelles for in vivo tumour-targeted delivery of artemisinin. Artificial Cells, Nanomed. Biotechnol. 47 (1), 104–114.
R. M. Dhahi, et al. (2016). Therapeutic targeting of anti-cancer drug Loaded PCL-PEG-PCL triblock copolymer nanoparticles toward MCF-7 breast cancer cell line. Int. J. Scientific Eng. Res. 7 (5), 1238–1244.
C. Hu, et al. (2017). Micelle or polymersome formation by PCL-PEG-PCL copolymers as drug delivery systems. Chinese Chem. Lett. 28 (9), 1905–1909.
K. Rostamizadeh, et al. (2018). Methotrexate-conjugated mPEG–PCL copolymers: a novel approach for dual triggered drug delivery. New J. Chem. 42 (8), 5937–5945.
H. R. K. Manjili, et al. (2016). Preparation and physicochemical characterization of biodegradable mPEG-PCL core-shell micelles for delivery of artemisinin. Pharm. Sci. 22 (4), 234–243.
B. Xiao, et al. (2015). Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J. Mater. Chem. B 3 (39), 7724–7733.
E. Fröhlich (2012). The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 7, 5577.
A. Kumari, S. K. Yadav, and S. C. Yadav (2010). Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and surfaces B: Biointerfaces 75 (1), 1–18.
M. Turner, G. Clough, and C. Michel (1983). The effects of cationised ferritin and native ferritin upon the filtration coefficient of single frog capillaries Evidence that proteins in the endothelial cell coat influence permeability. Microvascular Res 25 (2), 205–222.
K. Murugan, et al. (2015). Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int. J. Nanomed. 10, 2191.
S. Z. H. Rizvi, et al. (2019). Simvastatin-loaded solid lipid nanoparticles for enhanced anti-hyperlipidemic activity in hyperlipidemia animal model. Int. J. Pharm. 560, 136–143.
G. I. Harisa, A. H. Alomrani, and M. M. Badran (2017). Simvastatin-loaded nanostructured lipid carriers attenuate the atherogenic risk of erythrocytes in hyperlipidemic rats. Eu. J. Pharm. Sci. 96, 62–71.
Y. Wu, et al. (2015). Novel simvastatin-loaded nanoparticles based on cholic acid-core star-shaped PLGA for breast cancer treatment. J Biomed. Nanotechnol 11 (7), 1247–1260.
W. Chunyan and S. Valiyaveettil (2013). Correlation of biocapping agents with cytotoxic effects of silver nanoparticles on human tumor cells. RSC Adv. 3 (34), 14329–14338.
S.-T. Wang, et al. (2017). Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 8 (2), e2626–e2626.
Z. Ma, et al. (2019). Inhibitory effect of simvastatin in nasopharyngeal carcinoma cells. Experimental Therapeutic Med. 17 (6), 4477–4484.
S. Zohre, et al. (2014). Trichostatin A-induced apoptosis is mediated by Kruppel-like factor 4 in ovarian and lung cancer. Asian Pacific J. Cancer Prevention 15 (16), 6581–6586.
D. R. McIlwain, T. Berger, and T. W. Mak (2013). Caspase functions in cell death and disease. Cold Spring Harbor Perspect. Biol. 5 (4), a008656.
S. Amirsaadat, et al. (2021). Metformin and Silibinin co-loaded PLGA-PEG nanoparticles for effective combination therapy against human breast cancer cells. J Drug Delivery Sci Technol 61, 102107.
S. Kany, et al. (2018). Simvastatin exerts anticancer effects in osteosarcoma cell lines via geranylgeranylation and c-Jun activation. Int. J. Oncol 52 (4), 1285–1294.
M. Koyuturk, M. Ersoz, and N. Altiok (2007). Simvastatin induces apoptosis in human breast cancer cells: p53 and estrogen receptor independent pathway requiring signalling through JNK. Cancer Lett. 250 (2), 220–228.
C. Spampanato, et al. (2012). Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int. J. Oncol. 40 (4), 935–941.
H. Karlic, et al. (2017). Statin and bisphosphonate induce starvation in fast-growing cancer cell lines. International journal of molecular sciences 18 (9), 1982.
Acknowledgements
The authors express their sincere thanks to the pharmaceutical sciences research center of Ardabil University of Medical Sciences for financial support.
Author information
Authors and Affiliations
Contributions
The conception and the design of the study were performed by KN. The experiments were carried out by all authors. Data collection and analyses were conducted by MG and MD. The project was supervised by KN. The manuscript was drafted by MD, and revised by KN. All authors participated in the manuscript in the critical review process of the manuscript and approval of the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dadashpour, M., Ganjibakhsh, M., Mousazadeh, H. et al. Increased Pro-Apoptotic and Anti-Proliferative Activities of Simvastatin Encapsulated PCL-PEG Nanoparticles on Human Breast Cancer Adenocarcinoma Cells. J Clust Sci 34, 211–222 (2023). https://doi.org/10.1007/s10876-021-02217-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02217-y