Abstract
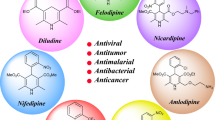
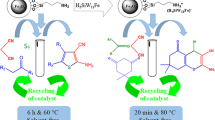
The green and effective procedure for synthesis of 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) was carried out from compound involving active methylene group, urea, and various aldehydes by using morpholinum sulphate salt immobilized onto magnetic nanoparticles as an efficient catalyst in ethanol solvent under ultrasound irradiation. Functionalized Fe3O4 as an effective and recyclable nanocatalyst was prepared and characterized using different methods including Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), vibrating sample magnetometry (VSM), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD) techniques. The advantage of this work is the easy separation of the nanocatalyst by using an external magnet, recyclability, non-toxicity, versatility and high stability of the catalyst. Also, low reaction times and excellent yields make the present protocol very useful and attractive for the synthesis of the titled products by ultrasonic irradiation. Furthermore, the products were identified using melting points, FT-IR and 1H NMR, spectroscopic data.












Similar content being viewed by others
References
P. Biginelli (1893). Gazz. Chim. Ital. 23, 360.
S. S. Panda, P. Khanna, and L. Khanna (2012). Curr. Org. Chem. 16, 507.
M. H. Majid, S. Asadi, and B. M. Boshra (2013). Mol. Diversity 17, 389.
C. D. Graaff, E. Ruijter, and R. V. A. Orru (2012). Chem. Soc. Rev. 41, 3969.
C. O. Kappe (2000). Eur. J. Med. Chem. 35, 1043.
L. H. S. Matos, F. T. Masson, L. A. Simeoni, and M. H. Mello (2018). Eur. J. Med. Chem. 143, 1779.
T. Peters, H. Lindenmaier, W. E. Haefeli, and J. Weiss (2006). Naunyn-Schmiedeberg’s Arch. Pharmacol. 372, 291.
J. C. Cochran, J. E. Gatial, T. M. Kapoor, and S. P. Gilbert (2005). J. Biol. Chem. 280, 12658.
S. Tcherniuk, R. V. Lis, F. Kozielski, and D. A. Skoufias (2010). Biochem. Pharmacol. 79, 864.
K. De, S. Chandra, B. Sarkar, S. Ganguly, and M. Misra (2010). J. Radioanal. Nucl. Chem. 283, 621.
J. Bais, F. Benedetti, F. Berti, I. Cerminara, S. Drioli, M. Funicello, G. Regini, M. Vidali, and F. Felluga (2020). Molecules 25, 4152.
S. H. Choi and D. McCollum (2012). Curr. Biol. 22, 225.
J. C. Cochran and S. P. Gilbert (2005). Biochem. 44, 16633.
L. Duan, T. Q. Wang, W. Bian, W. Liu, Y. Sun, and B. S. Yang (2015). Spectrochim. Acta. A 137, 1086.
S. DeBonis, J. P. Simorre, I. Crevel, L. Lebeau, D. A. Skoufias, A. Blangy, C. Ebel, P. Gans, R. Cross, D. D. Hackney, R. H. Wade, and F. Kozielski (2003). Biochem. 42, 338.
K. Drosopoulos, C. Tang, W. C. H. Chao, and S. Linardopoulos (2014). Nat. Commun. 5, 3686.
C. J. Funk, A. S. Davis, J. A. Hopkins, and K. M. Middleton (2004). Anal. Biochem. 329, 68.
R. V. Chikhale, R. P. Bhole, P. B. Khedekar, and K. P. Bhusari (2009). Eur. J. Med. Chem. 44, 3645.
S. R. Patil, A. S. Choudhary, V. S. Patil, and N. Sekar (2015). Fibers Polym. 16, 2349.
C. Boukis, A. Llevot, and M. A. R. Meier (2016). Macromol. Rapid. Commun. 37, 643.
Y. Zhao, Y. Yu, Y. Zhang, X. Wang, B. Yang, Y. Zhang, Q. Zhang, C. Fu, Y. Wei, and L. Tao (2015). Polym. Chem. 6, 4940.
A. Rahimi, R. Ghorbani-Vaghei, and S. Alavinia (2021). J. Porous Mat. 28, 1643.
S. Alavinia and R. Ghorbani-Vaghei (2021). Monatsh. Chem. 152, 1269.
F. Hamidi-Dastjerdi, R. Ghorbani-Vaghei, and S. Alavinia (2020). Catal. Lett. 150, 3514.
S. Alavinia and R. Ghorbani-Vaghei (2020). New J. Chem. 44, 13062.
A. S. Paraskar, G. K. Dewkar, and A. Sudalai (2003). Tetrahedron Lett. 44, 3305.
K. A. Kumar, M. Kasthuraiah, C. S. Reddy, and C. D. Reddy (2001). Tetrahedron Lett. 42, 7873.
M. Gohain, D. Prajapati, and J. S. Sandhu (2004). Synlett 2, 235.
T. Simon and J. Rodriguez. Constantieux (2004). Eur. J. Org. Chem. 2004, 4957.
J. S. Yadav, B. V. S. Reddy, P. Sridhar, J. S. S. Reddy, K. Nagaiah, N. Lingaiah, and P. S. Saiprasad (2004). Eur. J. Org. Chem. 2004, 552.
O. Kappe and S. F. Falsonev (1998). Synlett 7, 718.
S. Xue, Y. C. Shen, Y. L. Li, X. M. Shen, and Q. X. Guo (2002). Chin. J. Chem. 20, 385.
J. Lu and Y. Bai (2002). Synthesis 4, 466.
V. R. Choudhary, V. H. Tillu, V. S. Narkhede, H. B. Borate, and R. D. Wakharkar (2003). Catal. Commun. 4, 449.
S. E. Hankari, B. M. Pérez, P. Hesemann, A. Bouhaouss, and J. J. E. Moreau (2011). Chem. Commun. 47, 6704.
J. Safari, S. Gandomi-Ravandi (2014) J. Mol. Catal. A. Chem. 373: 72.
J. Safaei-Ghomi, R. Teymuri, and A. Ziarati (2013). Monatsh. Chem. 144, 1865.
H. R. Memarain and M. Ranjbar (2012). J. Mol. Catal. A. 356, 46.
J. Javidi, M. Esmaeilpour, and F. N. Dodeji (2014). RSC Adv. 5, 308.
B. K. Banik, A. T. Reddy, A. Datta, and C. Mukhopadhyay (2007). Tetrahedron Lett. 48, 7392.
J. Safari and S. Gandomi-Ravandi (2014). New. J. Chem. 38, 3514.
J. T. Li, J. F. Han, J. H. Yang, and T. S. Li (2003). Ultrason. Sonochem. 10, 119.
J. Peng and Y. Deng (2001). Tetrahedron Lett. 42, 5917.
A. Zhu, Q. Li, L. Li, and J. Wang (2013). Catal. Lett. 143, 463.
M. Rahman, A. Sarkar, M. Ghosh, A. Majee, and A. Hajra (2014). Tetrahedron Lett. 55, 235.
S. Verma, S. L. Jain, and B. Sain (2010). Tetrahedron Lett. 51, 6897.
T. Welton (1999). Chem. Rev. 99, 2071.
V. I. Pârvulescu and C. Hardacre (2007). Chem. Rev. 107, 2615.
B. C. Ranu and S. Banerjee (2005). Org. Lett. 7, 3049.
S. Alavinia and R. Ghorbani-Vaghei (2021). RSC Adv. 11, 29728.
M. Faraji, Y. Yamini, and M. Rezaee (2010). J. Iran. Chem. Soc. 7, 1.
S. Zafari, R. Ghorbani-Vaghei, and S. Alavinia (2021). Mat. Chem. Phys. 270, 124840.
S. J. Wang, Z. Y. Wang, and Z. G. Zha (2009). Dalton Trans. 43, 9363.
R. B. N. Baig and R. S. Varma (2013). Chem. Commun. 49, 752.
Q. Zhang, H. Su, J. Luo, and Y. Wei (2014). Green Chem. 14, 201.
G. Derouane, J. C. Védrine, P. R. Pinto, P. M. Borges, L. Costa, M. Lemos, F. Lemos, and F. R. Ribeiro (2013). Catal. Rev. 55, 454.
G. Cravotto and P. Cintas (2006). Chem. Soc. Rev. 35, 180.
T. J. Mason and J. Phillip, Applied sonochemistry-the uses of power ultrasound in chemistry and processing. (Wiley-VCH, Weinheim, 2002), pp. 43–167.
R. F. Abdulla (1988). Aldrichimica Acta 21, 31.
Z. Akbarzadeh and J. Safaei-Ghomi (2020). Green Chem. Lett. Rev. 13, 141.
B. Sreedhar and P. S. Reddy (2007). Synth. Commun. 37, 805.
H. Xu, B. W. Zeiger, and K. S. Suslick (2013). Chem. Soc. Rev. 42, 2555.
R. Ghahremanzadeh, Z. Rashid, A. H. Zarnani, and H. Naeimi (2014). Ultrason. Sonochem. 21, 1451.
Y. L. Pang and A. Z. Abdullah (2012). Ultrason. Sonochem. 19, 642.
S. Allahyari, M. Haghighi, A. Ebadi, and S. Hosseinzadeh (2014). Ultrason. Sonochem. 21, 663.
M. Mirza-Aghayan, N. Ganjbakhsh, M. M. Tavana, and R. Boukherroub (2016). Ultrason. Sonochem. 32, 37.
M. H. Valkenberg, C. Decastro, and W. F. Holderich (2002). Green. Chem. 4, 88.
S. A. Galema (1997). Chem. Soc. Rev. 26, 233.
M. Bagherzadeh, M. Haghdoost, and F. Matlobi-Moghaddam (2013). J. Coord. Chem. 66, 3025.
A. Shaabani, A. Bazgir, and F. Teimouri (2003). Tetrahedron Lett. 44, 857.
Azarifar, O. Badalkhani, Y. Abbasi (2016). J. Sulfur. Chem. 7, 1.
A. O. Kappe (1993). Tetrahedron 49, 6937.
Woerly, The Biginelli Reaction: Development and Applications, November 24, 2008.
S. Farooq, F. A. Alharthi, A. Alsalme, A. Hussain, B. A. Dar, A. Hamid, and S. Koul (2020). RSC Adv. 10, 42221.
N. C. Desai, S. B. Joshi, and K. A. Jadeja (2019). J. Heterocycl. Chem. 56, 1.
L. G. Nascimento, I. M. Dias, G. B. Souza, I. Dancini-Pontes, N. R. Fernandes, P. Souza, G. R. Oliveira, and C. G. Alonso (2020). J. Org. Chem. 85, 11170.
N. Popovics-Tóth, Á. Tajti, E. Hümpfner, and E. Bálint (2021). Catalysts 11, 45.
Acknowledgements
The authors are grateful to the University of Kashan for supporting this work by Grant No. 159148/90.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oboudatian, HS., Moradian, M. & Naeimi, H. Morpholinum Sulphate Salt Immobilized Onto Magnetic NPs Catalyzed Sonication Green Synthesis of Dihydropyrimidinones. J Clust Sci 34, 297–309 (2023). https://doi.org/10.1007/s10876-021-02214-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02214-1