Abstract
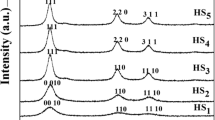
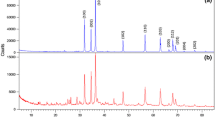
A novel and simple one-step solid state reaction in the presence of a suitable surfactant, sodium dodecyl sulfate (SDS), and a novel precursor, [bis(acetylacetonato)zinc(II)]; [Zn(acac)2]; has been developed to synthesize uniform zinc oxide microflakes with an average thickness of 0.3–2.4 μm. In the absence of SDS the product samples contained microrods. The formation of zinc oxide microflakes depends on the molar ratio of Zn(II)/SDS and the experimental procedure. The products were characterized by X-ray diffraction, photoluminescence spectroscopy, FT-IR spectroscopy, surface area, scanning electron microscopy and transmission electron microscopy to depict the phase and morphology. The synthesized ZnO microflakes have a hexagonal zincite structure.








Similar content being viewed by others
References:
A. D. Hardy, H. H. Sutherland, and R. Vaishnav (2002). J. Ethnophrma. 80, 137.
L. C. Sim, S. R. Ramanan, H. Ismail, K. N. Seetharama, and T. J. Goh (2005). Thermochim. Acta. 430, 155.
P. Sulcova and M. N. Trojan (1999). Dyes Pigment. 40, 83.
S. C. Singh and R. Gopal (2008). J. Phys. Chem. C 112, 2812.
J. Ya, C. Li, and S. Liu (2008). J.Colloid Interface.Sci. 326, 433.
L. Sikong, J. Damchan, K. Kooptarnond, and S. Niyomwas (2008). Songklanakarin J. Sci. Technol. 30, 385.
M. Vaface and M. S. Ghamsari (2007). Mater. Lett. 61, 3265.
J. M. Jang, C. R. Kim, H. Ryu, M. Razeghi, and W. G. Jung (2008). J. Alloys Compd. 463, 503.
M. Salavati-Niasari, F. Davar, and A. Khansari (2011). J. Alloys Compd. 509, 61.
M. Salavati-Niasari, A. Khansari, and F. Davar (2009). Inorganica. Chimica. Acta. 362, 4937.
M. Salavati-Niasari, N. Mir, and F. Davar (2010). J. Alloys Compd. 493, 163.
F. Davar, Z. Fereshteh, and M. Salavati-Niasari (2009). J. Alloys Compd. 476, 797.
M. Salavati-Niasari, N. Mir, and F. Davar (2010). Appl. Surf. Sci. 256, 4003.
M. Salavati-Niasari, F. Davar, and Z. Fereshteh (2010). J. Alloys Compd. 494, 410.
A. Askarinejad, M. A. Alavi, and A. Morsali (2011). Iran J. Chem. Chem. Eng. 30, 75.
S. Suwanboon, P. Amornpitoksuk, A. Haidoux, and J.-C. Tedenac (2008). J. Alloys Compd. 462, 335.
S. Liufu, H. Xiao, and Y. Li (2004). Powder Technol. B 145, 20.
C. Jin, X. Yuan, W. Ge, J. Hong, and X. Xin (2003). Nanotechnology 14, 667.
Y. W. Zhang, M. Tang, and X. Jin (2003). Solid State Sci. 5, 435.
G. Sun, M. Cao, Y. Wang, C. Hu, Y. Liu, L. Ren, and Z. Pu (2006). Mater. Lett. 60, 2777.
H. Yin, Z. Xu, G. Wang, J. Bai, and H. Bao (2005). Mater. Chem. Phys. 91, 130.
R. G. Charles and M. A. Pawlikowski (1958). J. Phys. Chem. 62, 440.
Z. P. Sun, L. Liu, L. Zhang, and D. Z. Jia (2006). Nanotechnology 17, 2266.
H. M. Ismail (1991). J. Anal. Appl. Pyrolysis 21, 315.
E. Hammarberg, A. Prodi-Schwab, and C. Feldmann (2009). J. Colloid Interface Sci. 334, 29.
Acknowledgments
Authors are grateful to the council of Iran National Science Foundation and University of Kashan for supporting this study by Grant No (159271/74).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salavati-Niasari, M., Gholami-Daghian, M., Esmaeili-Zare, M. et al. Solid State Synthesis and Characterization of Zinc Oxide (ZnO) Microflakes by [Bis(acetylacetonato)zinc(II)] and Sodium Hydroxide at Room Temperature. J Clust Sci 24, 1093–1101 (2013). https://doi.org/10.1007/s10876-013-0600-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0600-5