Abstract
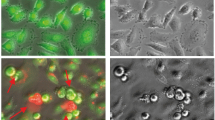
Arene ruthenium complexes containing long-chain N-ligands L1 = NC5H4–4-COO–C6H4–4-O–(CH2)9–CH3 or L2 = NC5H4–4-COO–(CH2)10–O–C6H4–4-COO–C6H4–4-C6H4–4-CN derived from isonicotinic acid, of the type [(arene)Ru(L)Cl2] (arene = C6H6, L = L1: 1; arene = p-MeC6H4Pri, L = L1: 2; arene = C6Me6, L = L1: 3; arene = C6H6, L = L2: 4; arene = p-MeC6H4Pri, L = L2: 5; arene = C6Me6, L = L2: 6) have been synthesized from the corresponding [(arene)RuCl2]2 precursor with the long-chain N-ligand L in dichloromethane. Ruthenium nanoparticles stabilized by L1 have been prepared by the solvent-free reduction of 1 with hydrogen or by reducing [(arene)Ru(H2O)3]SO4 in ethanol in the presence of L1 with hydrogen. These complexes and nanoparticles show a high anticancer activity towards human ovarian cell lines, the highest cytotoxicity being obtained for complex 2 (IC50 = 2 μM for A2780 and 7 μM for A2780cisR).





Similar content being viewed by others
References
G. Süss-Fink (2010). Dalton Trans. 39, 1673.
P. J. Dyson (2007). Chimia. 61, 698.
S. J. Dougan and P. J. Sadler (2007). Chimia. 61, 704.
L. D. Dale, J. H. Tocher, T. M. Dyson, D. I. Edwards, and D. A. Tocher (1992). Anti-Cancer Drug Design. 7, 3.
C. S. Allardyce, P. J. Dyson, D. J. Ellis, and S. L. Heath (2001). Chem. Commun. 1396.
R. E. Morris, R. E. Aird, P. d. S. Murdoch, H. Chen, J. Cummings, N. D. Hughes, S. Pearsons, A. Parkin, G. Boyd, D. I. Jodrell, and P. J. Sadler (2001). J. Med. Chem. 44, 3616.
G. V. Tsarichenko, V. I. Bobrov, and M. V. Smarkov (1977). Pharm. Chem. J. 11, 481.
J. Suarez, K. Ranguelova, and A. A. Jarzecki (2009). J. Biol. Chem. 284, 7017.
J. G. Małecki, R. Kruszynski, M. Jaworska, P. Lodowski, and Z. Mazurak (2008). J. Organomet. Chem. 693, 1096.
T. Arthur and T. A. Stephenson (1981). J. Organomet. Chem. 208, 369.
M. A. Bennett, G. B. Robertson, and A. K. Smith (1972). J. Organomet. Chem. 43, C41.
M. A. Bennett, T. W. Matheson, G. B. Robertson, A. K. Smith, and P. A. Tucker (1980). Inorg. Chem. 10, 1014.
M. S. Röthlisberger, W. Hummel, P.-A. Pittet, H.-B. Bürgi, A. Ludi, and A. E. Merbach (1988). Inorg. Chem. 27, 1358.
W. Weber and P. C. Ford (1986). Inorg. Chem. 25, 1088.
S. Ogo, T. Abura, and Y. Watanabe (2002). Organometallics. 21, 2964.
M. Marcos, M. B. Ros, J. L. Serrano, M. A. Esteruelas, E. Sola, L. A. Oro, and J. Barberà (1990). Chem. Mater. 2, 748.
B. Dardel, D. Guillon, B. Heinrich, and R. Deschenaux (2001). J. Mater. Chem. 11, 2814.
M. D. Abramoff, P. J. Magelhaes, and S. J. Ram (2004). Biophotonics Int. 11, 36.
Y. Malam, M. Loizidou, and A. M. Seifalian (2009). Trends Pharm. Sci. 30, 592.
P. P. Jumade, A. M. Gupta, P. W. Dhore, P. S. Wake, V. V. Pande, and A. Deshmukh (2009). J. Chem. Pharm. Sci. 2, 158.
M. E. Gindy and R. K. Prud’homme (2009). Exp. Opin. Drug Deliv. 6, 865.
W. Lin, T. Hyeon, G. M. Lanza, M. Zhang, and T. J. Meade (2009). MRS Bull. 34, 441.
D. F. Baban and L. W. Seymour (1998). Adv. Drug Deliv. Rev. 34, 109.
M. Vaccaro, R. Del Litto, G. Mangiapia, A. M. Carnerup, G. D’Errico, F. Ruffoa, and L. Paduano (2009). Chem. Commun. 1404.
W. H. Ang and P. J. Dyson (2006). Eur. J. Inorg. Chem. 4003.
F. Schmitt, P. Govindaswamy, G. Süss-Fink, W. H. Ang, P. J. Dyson, L. Juillerat-Jeanneret, and B. Therrien (2008). J. Med. Chem. 51, 1811.
P. Govender, N. C. Antonels, J. Mattsson, A. K. Renfrew, P. J. Dyson, J. R. Moss, B. Therrien, and G. S. Smith (2009). J. Organomet. Chem. 694, 3470.
J. Mattsson, P. Govindaswamy, A. K. Renfrew, P. J. Dyson, P. Štěpnička, G. Süss-Fink, and Bruno. Therrien (2009). Organometallics. 28, 4350.
M. G. Mendoza-Ferri, C. G. Hartinger, A. A. Nazarov, R. E. Eichinger, M. A. Jakupec, K. Severin, and B. K. Keppler (2009). Organometallics. 28, 6260.
B. Therrien, W. H. Ang, F. Chérioux, L. Vieille-Petit, L. Juillerat-Jeanneret, G. Süss-Fink, and P. J. Dyson (2007). J. Clust. Sci. 18, 741.
M. Auzias, B. Therrien, G. Süss-Fink, P. Štěpnička, W. H. Ang, and P. J. Dyson (2008). Inorg. Chem. 47, 578.
C. A. Vock, C. Scolaro, A. D. Phillips, R. Scopelliti, G. Sava, and P. J. Dyson (2006). J. Med. Chem. 49, 5552.
K. Furuta, H. Shirahashi, H. Yamashita, K. Ashibe, and E. Kuwano (2006). Biosci. Biotechnol. Biochem. 70, 746.
W. G. Friebe, W. Kampe, M. Linssen, and O. H. Wilhelms (1992). Ger. Offen. DE 4038335 A1 19920604.
D. S. Goldfarb (2009) U.S. Patent Appl. Publ. US 2009163545 A1 20090625.
Acknowledgments
Financial support of this work from the Fonds National Suisse de la Recherche Scientifique (Grant no. 200021-115821) is gratefully acknowledged. We also thank the Johnson Matthey Research Centre for a generous loan of ruthenium(III) chloride hydrate.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to Professor Malcolm Chisholm on the occasion of his 65th birthday.
Rights and permissions
About this article
Cite this article
Süss-Fink, G., Khan, FA., Juillerat-Jeanneret, L. et al. Synthesis and Anticancer Activity of Long-Chain Isonicotinic Ester Ligand-Containing Arene Ruthenium Complexes and Nanoparticles. J Clust Sci 21, 313–324 (2010). https://doi.org/10.1007/s10876-010-0298-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-010-0298-6