Abstract
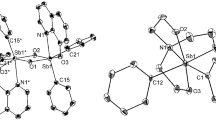
A series of three new compounds obtained from the reaction of Rh2(OAc)4 and 2, 2′ -dipyridylamine (Hdpa) under various conditions have been characterized. All are diamagnetic and have a Rh–Rh single bond. In Rh2(dpa)4, 1, there are four bridging dpa anions which bind the two Rh atoms through one pyridyl N atom and one amido N atom though two of these ligands interact further with a rhodium atom through the third N atom. In the other two compounds the Hdpa ligand is neutral. Thus Rh2(OAc)4(Hdpa)2, 2, is an adduct of the well known complex dirhodium tetraacetate in which the two Hdpa ligands occupy axial positions. In the third compound, Rh2(Hdpa)2(OAc)2Cl2, 3, only two acetate bridges are present. One Hdpa molecule chelates equatorially each rhodium atom and the chloride ions are axially coordinated. The Rh–Rh distances are 2. 4005(6) and 2. 4042(8) Å for 1 and 2, respectively. For 3, the Rh–Rh distance of 2. 593(1) Å is significantly longer than those in 1 and 2 because of the presence of fewer bridging ligands.
Similar content being viewed by others
REFERENCES
(a)F. A. Cotton, L. M. Daniels, C. A. Murillo, and I. Pascual (1997). J. Am. Chem. Soc. 119, 10223. (b)R. Cle ´rac, F. A. Cotton, L. M. Daniels, K. R. Dunbar, C. A. Murillo, and I. Pascual (2000). Inorg. Chem. 39, 748.
(a)E. -C. Yang, M. -C. Cheng, M. -S. Tsai, and S. -M. Peng (1994). J. Chem. Soc., Chem. Commun. 20, 2377. (b)R. Cle ´rac, F. A. Cotton, L. M. Daniels, K. R. Dunbar, K. Kirschbaum, C. A. Murillo, A. A. Pinkerton, A. J. Schultz, and X. Wang (2000). J. Am. Chem. Soc. 122, 6226.
(a)S. Aduldecha and B. Hathaway (1991). J. Chem. Soc., Dalton Trans. 993. (b) R. Cle ´rac, F. A. Cotton, K. R. Dunbar, C. A. Murillo, I. Pascual, and X. Wang (1999). Inorg. Chem. 38, 2655.
(a)L. -P. Wu, P. Field, T. Morrissey, P. Nagle, B. Hathaway, C. Simmons, and P. Thornton (1990). J. Chem. Soc., Dalton Trans. 3853. (b)G. J. Pyrka, M. El-Mekki, and A. A. Pinkerton (1991). J. Chem. Soc., Chem. Commun. 84. (c)J. F. Berry, F. A. Cot-ton, P. Lei, and C. A. Murillo (2003). Inorg. Chem. 42, 377.
J. -T. Sheu, C. -C. Lin, I. Chao, C. -C. Wang, and S. -M. Peng (1996). Chem. Commun. 3, 315.
J. F. Berry, F. A. Cotton, and C. A. Murillo (2004). Inorg. Chim. Acta In press.
J. F. Berry, F. A. Cotton, L. M. Daniels, C. A. Murillo, and X. Wang (2003). Inorg. Chem. 42, 2418 and references therein.
G. A. Rempel, P. Legzdins, H. Smith, and G. Wilkinson (1972). Inorg. Synth. 13, 90.
(a)A. Messerschmidt and J. Pflugrath (1987). J. Appl. Crystallogr. 20, 306. Pflugrath, J. Messerschmidt, A. MADNES, Munich Area Detector (New EEC)System, Version EEC 11/1/89, with enhancements by Enraf-Nonius Corp., Delft, The Netherlands.
(a)W. Kabsch (1988). J. Appl. Crystallogr. 21, 67. (b)W. Kabsch (1988). J. Appl. Crys-tallogr. 21, 916.
Program for absorption correction for Enraf-Nonius FAST diffractometer using the method of R. H. Blessing (1995). Acta. Crystallogr. A51, 33.
SHELXTL (1994). Version 5. 03. Siemens Industrial Automation Inc. Madison, WI, USA.
F. A. Cotton, C. A. Murillo, and S. -E. Stiriba (1999). Inorg. Chem. Commun. 2, 463.
(a)J. L. Bear, C. -L. Yao, L. -M. Liu, F. J. Capdevielle, J. D. Korp, T. A. Albright, S. -K. Kang, and K. M. Kadish (1989). Inorg. Chem. 28, 1254. (b)F. A. Cotton, P. Lei, C. Lin, C. A. Murillo, X. Wang, S. -Y. Yu, and Z. -X. Zhang (2004). J. Am. Chem. Soc. 126, 1518 and references therein.
F. A. Cotton, L. M. Daniels, C. A. Murillo, I. Pascual, and H. -C. Zhou (1999). J. Am. Chem. Soc. 121, 6856.
C. M. Kepert, G. B. Deacon, and L. Spiccia (2003). Inorg. Chim. Acta 355, 213.
F. A. Cotton and R. A. Walton (1992). Multiple Bonds Between Metal Atoms, Oxford University Press, Oxford.
A. P. Kochetkova, L. B. Sveshnikova, V. M. Stepanovich, and V. I. Sokol (1982). Koord. Khim. 8, 529.
E. Galdecka, Z. Galdecki, F. P. Pruchnik, and P. Jakimowicz (2000). Transition Met. Chem. 25, 315.
C. A. Crawford, J. H. Matonic, J. C. Huffman, K. Folting, K. R. Dunbar, and G. Christou (1997). Inorg. Chem. 36, 2361.
F. P. Pruchnik, P. Jakimowicz, Z. Ciunik, L. A. Oro, C. Tejel, and M. A. Ciriano (2001). Inorg. Chem. Commun. 4, 19.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berry, J.F., Cotton, F.A., Lin, C. et al. Exploring the Reactivity of Rh2 (OAc)4 with 2, 2′ -Dipyridylamine. Journal of Cluster Science 15, 531–541 (2004). https://doi.org/10.1007/s10876-004-5773-5
Issue Date:
DOI: https://doi.org/10.1007/s10876-004-5773-5