Abstract
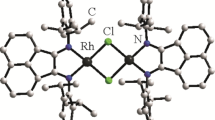
The rhodium diacetate complexes with dioxane Rh2(OAc)4(Diox)2 (I) and dimethyl sulfoxide Rh2(OAc)4(Dmso)2 (II) have been synthesized by dissolving rhodium diacetate [Rh2(OAc)4] in dioxane (Diox) and dimethyl sulfoxide (Dmso) with the further slow evaporation of the solvent. Rh2(OAc)4[(4-FC6H4)3Sb]2 (III) has been synthesized by the reaction between [Rh2(OAc)4] and tris-4-fluorophenylantimony in acetonitrile. Using X-ray diffraction, it has been established that the rhodium atoms in complexes I–III have a slightly distorted octahedral coordination: the angles ORhRh, ORhO, 176.56(6)°–177.72(4)° (I); SRhRh, ORhO, 174.79(9)°–179.271(13)° (II); SbRhRh, ORhO 173.590(19)°–175.48(10)° (III); bonds Rh–Rh, Rh–OAc, Rh–ODiox, 2.380(3), 2.037(3)–2.046(3), 2.335(3) Å (I); Rh–Rh, Rh–O, Rh–S, 2.4288(10), 2.034(3)–2.046(3), 2.7258(10) Å (II); Rh–Rh, Rh–O, Rh–Sb, 2.4183(12), 2.033(3)–2.044(3), 2.7113(13)–2.7120(13) Å (III). The structural organization in crystals of complexes I–III is caused by weak hydrogen bonds Н···ODiox (2.50–2.72 Å), Н···OAc (2.57 Å) (I), Н···ODmso 2.48–2.71 Å, Н···OAc 2.65, 2.66 Å (II) H···F (2.56–2.62 Å), and H···O (2.68–2.71 Å) (III).



Similar content being viewed by others
REFERENCES
D. D. Makitova, O. N. Krasochka, L. O. Atovmyan, et al., Koord. Khim. 13, 383 (1987).
J. Graf and W. Frank, Z. Anorg. Allg. Chem. 630, 1894 (2004). https://doi.org/10.1002/zaac.200400208
V. I. Pekhnyo, S. I. Orysyk, V. V. Bon, et al., Pol. J. Chem. 80, 1767 (2006).
M. Bujak and W. Frank, Z. Kristallogr.—New Cryst. Struct. 229, 147 (2014). https://doi.org/10.1515/ncrs-2014-0083
M. Bujak and W. Frank, Z. Naturforsch., B: Chem. Sci. 57, 1391 (2002).
M. Bujak, Cryst. Growth Des. 15, 1295 (2015). https://doi.org/10.1021/cg501694d
E. Alessio, A. S. Santi, P. Faleschini, et al., J. Chem. Soc., Dalton Trans., 1849 (1994). https://doi.org/10.1039/DT9940001849
A. Abbasi, S. Geranmayeh, M. Y. Skripkin, et al., Dalton Trans. 41, 850 (2012). https://doi.org/10.1039/C1DT11698C
V. V. Sharutin, O. K. Sharutina, V. S. Senchurin, and N. V. Somov, Russ. J. Coord. Chem. 40, 821 (2014). https://doi.org/10.1134/S1070328414110074
V. V. Sharutin, O. K. Sharutina, and V. S. Senchurin, Russ. J. Gen. Chem. 86, 2141 (2 016). https://doi.org/10.1134/S1070363216090309
Yu. S. Varshavskii, T. G. Cherkasova, V. N. Khrustalev, et al., Russ. J. Coord. Chem. 33, 194 (2007). https://doi.org/10.1134/S1070328407030074
F. A. Cotton and D. A. Ucko, Inorg. Chim. Acta 6, 161 (1972). https://doi.org/10.1016/S0020-1693(00)91778-X
G. W. Adamson, J. J. Daly, and D. Forster, J. Organomet. Chem. 71, C17 (1974). https://doi.org/10.1016/S0022-328X(00)93156-5
D. H. Nguyen, N. Lassauque, L. Vendier, et al., Eur. J. Inorg. Chem., No. 2, 326 (2014). https://doi.org/10.1002/ejic.201300933
P. Mura, J. Coord. Chem. 48, 503 (1999). https://doi.org/10.1080/00958979908023590
L. Vigo, M. J. Poropudas, P. Salin, et al., J. Organomet. Chem. 694, 2053 (2009). https://doi.org/10.1016/j.jorganchem.2009.02.001
S. E. Boyd, L. D. Field, and T. W. Hambley, Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 50, 1019 (1994). https://doi.org/10.1107/S010827019201271X
F. A. Cotton, B. G. DeBoer, M. D. LaPrade, et al., Acta Crystallogr., Sect. B: Struct. Sci. 27, 1664 (1971). https://doi.org/10.1107/S0567740871004527
JuniorD. S. Martin, T. R. Webb, G. A. Robbins, et al., Inorg. Chem. 18, 475 (1979). https://doi.org/10.1021/ic50192a061
M. Moszner, T. Glowiak, and J. J. Ziolkowski, Polyhedron 4, 1413 (1985). https://doi.org/10.1016/S0277-5387(00)86972-7
V. Noinville, B. Viossat, and N.-H. Dung, Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 49, 1297 (1993). https://doi.org/10.1107/S0108270193000022
F. A. Cotton and T. R. Felthouse, Inorg. Chem. 19, 323 (1980). https://doi.org/10.1021/ic50204a010
G. G. Christoph and Y.-B. Koh, J. Am. Chem. Soc. 101, 1422 (1979). https://doi.org/10.1021/ja00500a011
R. J. H. Clark, A. J. Hempleman, H. M. Dawes, et al., J. Chem. Soc., Dalton Trans., I775 (1985). https://doi.org/10.1039/DT9850001775
SMART and SAINT-Plus: Data Collection and Processing Software for the SMART System, Versions 5.0 (Bruker, Madison, WI, USA, 1998).
SHELXTL/PC: An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data, Vers. 5.10 (Bruker, Madison, WI, USA, 1998).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
S. S. Batsanov, Russ. J. Inorg. Chem. 36, 3015 (1991).
Yu. A. Fialkov, Not Only in Water (Khimiya, Leningrad, 1989) [in Russian].
M. Mantina, A. C. Chamberlin, R. Valero, et al., J. Phys. Chem. A 113, 5806 (2009). https://doi.org/10.1021/jp8111556
Funding
The National South Ural State Research University is grateful to the Ministry of Education and Science of the Russian Federation for financial support (grant no. 4.6151.2017/8.9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K. & Senchurin, V.S. Rhodium(II) Acetate Donor–Acceptor Complexes Rh2(OAc)4(Diox)2, Rh2(OAc)4(Dmso)2, and Rh2(OAc)4[(4-FC6H4)3Sb]2: Synthesis and Structure. Russ. J. Inorg. Chem. 64, 1025–1030 (2019). https://doi.org/10.1134/S0036023619080138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619080138