Abstract
Introduction
Mucosal lymphoid changes were observed in cryopreserved rectal tissues obtained from BALB/c mice infected with Shigella dysenteriae 1, immunized with 57-kDa major antigenic outer membrane protein, and infection after immunization.
Discussion
Our data suggested that caspase-3 is downregulated in CD4+ cells of immunized BALB/c mice following infection with substantial increased expression of interleukin (IL)-2 and interferon (IFN)-γ, while caspase-1 is upregulated in CD8+ cells with decreased expression of IL-4 and IL-10. This indicated an involvement of Fas-mediated lytic pathway for selective deletion of CD8+ cells out of CD3+ T cells. IL-18 promotes inflammation and induces IFN-γ and tumor necrosis factor (TNF)-α as the expression of IFN-γ and TNF-α cytokines was evident in this study. It is assumed that the role of caspase-1 in inducing the CD4+ T cell activity increased with IL-18 rather than CD8+ suppressor cell activity. Bcl-2 is capable of inhibiting the Fas/Fas-L-mediated cell death for helper cells. Overall, the findings indicate that majority of the apoptotic cells were CD8+ T cells in the groups of infection following immunization, and there might be a selective deletion of T lymphocytes mediated by caspase-1 via IL-18.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In shigellosis, the progression of the disease toward severity is by three main processes—invasion of enterocytes and entry through M cells [1, 2], multiplication and intracellular spreading [3, 4], and finally by massive infiltration of mucosal enterocytes, lymphocytes, and natural killer cells [5, 6]. The pathogenesis of Shigella depends on the virulence of the organism as well as host resistance factors. The virulence of mucosal pathogens would preliminarily depend on the inflammatory responses that they induce. Once Shigella escapes from mucosal immune defense, they enter the epithelial cells and trigger a series of inflammatory mediators [7]. They cross the mucosa and activate macrophages and dendritic cells [8]. Lymphocytes were predominantly found in stool samples [9] as well as in peripheral blood [10] of Shigella-infected patients.
Pathogenesis involves both apoptosis and necrosis of the epithelial mucosa and lamina propria lymphoid cells [1, 11]. Many inflammatory reactions have been demonstrated during pathomechanism leading to necrotic lesions of mucosal tissue [11, 12]. Macrophage apoptosis is mediated by Shigella IpaB protein after coupling with intracellular proteases via caspase-1 activation [13] leading to the generation of interleukin (IL)-1 cytokines [12, 14]. This activation results in cleavage of pro-inflammatory cytokines into mature IL-1β and IL-18 [15, 16]. The IL-1β and IL-18 are initially activated by caspase-1 protein essential for acute inflammation during Shigella flexneri infection in caspase-1−/− knockout mice [17]. In vitro experiments have described the possible role of cytokines like IL-1β and tumor necrosis factor (TNF)-α in hemolytic–uremic syndrome [18]. On the other hand, the role of TNF-α was also evident in induction of neutrophil and eosinophil cell apoptosis by ligation of Fas receptors [19, 20]. Further, inflammatory responses occurred during the pathogenesis of shigellosis in response to acquire host immunity. Unlike other cytokines, downregulation of interferon (IFN)-γ and IL-2 was detected in the acute phase and increased gradually at the convalescent stages of day 14 and day 30 due to downmodulation of their receptors [21–23] or due to upregulation of Fas/Fas-L [24]. This might be due to lack of selective T cells although there is a majority of CD3+ apoptotic cells. Induction of T cell-mediated death pathways by perforin, granzyme, and Fas/Fas-L cytolytic molecules is prevalent, and the downregulation of cell survival promoters, Bcl-2 and IL-2, were studied in acute shigellosis [24]. Infections [25, 26] or vaccinations [27] are usually followed by an antibody response against the outer membrane protein (OMP), lipopolysaccharide (LPS), and invasive proteins (Ipa-s) at the mucosal sites [28, 29]. The intensity of antibody response at the periphery as well as mucosal sites was shown to correlate with the severity of infection [30, 31]. Recent observations have indicated that the Ipa proteins induce apoptosis by suppressing the signaling pathways leading to production of TNF-α, essential for the control of this infection [13].
Earlier, we have reported the role of 57-kDa major antigen in inducing major histocompatibility complex class-II restricted T cell responses in vitro to recognize the Shigella-specific epitopes to acquire host defense against the infection [32]. In the present study, an effort was made to observe the in vivo behavioral changes in mucosal lymphoid of the cryopreserved rectal tissues obtained from BALB/c mice infected with Shigella dysenteriae 1, immunized with 57-kDa major antigen, infection after immunization with a lethal dose of bacteria and healthy control. The rationale behind this study is to understand the mechanism of lymphoid cell apoptosis following infection with a lethal dose of S. dysenteriae 1 before and after immunization. We observed that Shigella 57-kDa antigenic OMP induced specific CD8+ T cell apoptosis mediated by caspase-1via IL-18.
Materials and Methods
Bacterial Culture and Isolation of 57-kDa Major Antigenic OMP
Serovar-specific strain of S. dysenteriae type 1 was isolated and identified from the feces of patients with bacillary dysentery. Bacteriological examination of stool was done by standard techniques [33]. After guinea pig passage as described by Sereny [34], virulent smooth colonies were grown at 37°C in Tryptic soy broth (Difco, USA). OMPs were isolated using a standard procedure as described by Johnston and Gotschlich [35], from S. dysenteriae type 1. In brief, after harvesting the cells from culture, they were sonicated and treated with sarkosyl (sodium lauroyl sarcosinate, 1% (w/v), Sigma, USA) for 30 min at 24°C, to selectively dissolve the inner membrane and centrifuged at 100,000×g for 2 h. The pellet containing OMP was washed with distilled water and stored at −20°C. The major antigenic fraction (57 kDa) was eluted from the gel slices electrophoretically using an electro-eluter (Bio-Rad, USA), as described in details earlier [36]. The protein was concentrated using Speed-Vac (Savant, USA), and concentration was measured using 1% bovine serum albumin (BSA) as standard as described by Markwell et al. [37]. To ascertain whether the eluted protein contained trace amounts of bound LPS, the Limulus amoebocyte lysate (Sigma, USA) assay was performed using Escherichia coli O55:B5 LPS (Sigma, USA) as control [38]; 10 pg/10 μg of LPS was detected which did not show any interference with the results.
Immunization and Challenge
Inbred male 3- to 4-month-old BALB/c mice were housed each in the animal unit of this institute according to the institutional guidelines. They were grouped into (a) infected, (b) immunized, (c) infection after immunization, and (d) control, groups each containing five mice. The second and third groups were immunized subcutaneously with 25 μg of the 57-kDa antigen emulsified in Freunds’ incomplete adjuvant at first, second, and third weeks, followed by a booster dose of 50 μg of antigen at the fifth week. After 10 days, animals of groups 1 and 3 were fed on glucose–water and were challenged with a 50% lethal dose (LD50) of 1 × 104 cfu/ml bacteria (S. dysenteriae type 1; strain PB10) orally [39]. The challenged mice were monitored for mortality. LD50 of the infection was determined by plotting percent mortality against dose using the software GraphPad.
Specific Antibody Responses Against Infection and Immunization
The serum antibody response for immunoglobulin (Ig)A and IgG was measured using enzyme-linked immunosorbent assay [40] subsequently on days 3, 10, 21, and 28 in immunized mice with respect to day 0 for control group. The same responses were also observed in challenged or challenged–immunized mice at days 1, 14, 21, and 28. The serum antibody responses were measured as the inverse log [ln (x)] of titer value measured at 492 nm.
Cryosectioning and Immunostaining
Mucosal lymphoid changes in response to the 57-kDa major antigenic OMP of S. dysenteriae 1 were studied in cryopreserved rectal tissue. The rectal tissues were collected from S. dysenteriae 1-infected and control mice in Histocon (Histolab, Sweden). Rectal tissue was also collected from 57-kDa antigen-immunized mice and followed with a lethal dose of homologous strain. The tissue was washed thrice with sterile cold phosphate-buffered saline (PBS; 1×; pH 7.4), snap-frozen in liquid nitrogen, and stored at −70°C until used for cryostat sectioning (Leica; CM1850, Germany). Frozen rectal tissue was sectioned at a thickness of 6 μm and mounted on glass slides. As described earlier by Raqib et al. [24], these sections were fixed with freshly prepared 2% acetone, rinsed in sterile PBS for 10 min followed by a few washes with Ca2+ and Mg2+ containing buffered saline solution (BSS) supplemented with 0.01 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (Gibco, USA). After washing, slides were allowed to dry and kept at −20°C until used. For apoptosis and caspase protein activation study, mounted slides were stored without fixation at −70°C until used.
Immunohistochemical Staining for Caspase Protein Markers and Cytokines
Immunohistochemical staining for cytokines and caspases protein markers were performed by in situ immunofluorescence procedure using Caspa-Tag fluorescein Caspase activity kit (S7303 and S7301; Intergen, Serologicals Co., USA). Briefly, the cryopreserved frozen slides were rehydrated in BSS followed by trypsinization (1% trypsin in BSS) for 30 min. After washing thrice with washing buffer, tissue sections were incubated for 1 h at 37°C with specific carboxyfluorescein (FAM)-peptide-fluoromethyl ketone (FMK) inhibitors at a concentration of 10 μl. To label the nuclei, a 5-μl/ml concentration of Hoechest stain was added. The tissue sections were washed twice with washing buffer. For the expression of cytokines, nonspecific binding sites of trypsinized tissue sections were blocked with 2% BSA and washed twice with washing buffer. Tissue sections were then incubated for 30 min at 37°C with antimouse-specific antibodies conjugated to fluorescein isothiocyanate (FITC; IL-2, IFN-γ, IL-18) or phycoerythrin (PE; IL-4, IL-10, TNF-α) at a concentration of 2–5 μg/ml in BSS. Finally, the tissue sections were washed with BSS and mounted in 1:1 phosphate-buffered glycerol.
Detection of Phenotypic T Cell Markers in Tissues
Immunophenotyping expression of T cell markers was detected in the tissue sections using immunofluorescent study. The cryosections were allowed to rehydrate in BSS and were incubated with 2% BSA for 30 min at 37°C to block the nonspecific sites and washed twice with washing buffer. The cells were then incubated with 50 μl of FITC-conjugated anti-CD4, anti-CD8, or anti-CD45 and PE-conjugated anti-CD3 antibody (Dako, Denmark) in duplicates for 30 min at 37°C. Finally, the cells were washed twice with PBS-T20 containing 1% BSA.
Detection of Phenotypic Apoptotic Cell Markers in Tissues
Early stages of apoptotic cells in unfixed tissues were visualized by the annexin V labeling method using the ApoTest-FITC in situ immunofluorescence apoptosis detection kit (K235011; Nexins Research, USA). The cryopreserved frozen slides were brought to room temperature, rehydrated in BSS. Nonspecific binding sites were blocked using binding buffer (1×) and incubated for 30 min at 37°C. After washing with washing buffer twice for 5 min each, the slides were incubated for 10 min on ice with antimouse annexin V-FITC antibody at a concentration of 2.5 μg/ml in BSS containing 0.1% triton-X100 and 2–5 μl of propidium iodide (2.5 mg/ml concentration) in the dark. Slides were washed twice with washing buffer for 5 min and mounted in 1:1 phosphate-buffered glycerol.
Image Analysis
Immunostaining of cell surface markers, cytokines, or apoptotic protein markers were examined with Olympus microscope (A051; Tokyo, Japan) equipped with a 3CCD color camera (Olympus DP11, Japan). The images were then examined in Camedia Master 1.2 image analyzer using data operating system. The positive cells as well as the negative cells were set as standard. Positive staining was defined by computer-assisted analysis of video microscopic images. The intensity of positive immunoreactivity was measured and expressed in 256 Gy level of the blue part, of the green part, and blue video signals. The acquired images were divided into 1,712 × 1,368 pixels, and each pixel was expressed in square micrometers after calibration with the current magnification [41]. The data acquired was imported to Microsoft Excel (MicroImage32). For each stained slide, three different areas of 100 μm2 field were read from three different sections of the slide to avoid any bias. The average of the three readings was used for each staining. The results were expressed as the percentage of the ratio of positive pixels to total pixels.
Statistical Analysis
Two-way analysis of variance and Student t test were performed to compare the effect of stimulants for each variable in infected, immunized following Shigella challenge, and in control group of mice. Wherever variance is not applicable, results were expressed as a mean ± SE for significance in t test. χ 2 tests were also applied to find probability (P) of association between two sets of events. The data for immunoassays were processed using a software package (Epistat) to generate a curve using linear regression analysis.
Results
Serum IgA and IgG Responses
The serum IgA and IgG levels in immunized and challenged–immunized BALB/c mice were significantly elevated (P < 0.05). After 2 weeks of infection, the level of IgA was found to decrease sixfold as compared to serum IgG level, though it was above the baseline titer observed before infection (data not shown).
Shigella Survival Rate in BALB/c Mice
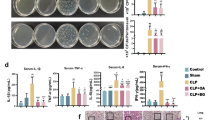
The BALB/c mice were challenged before and after 57-kDa antigen immunization with a LD50 of 104 cfu/ml S. dysenteriae 1. The percent mortality was twofold higher in LD50-infected mice than that of the LD50-infected immunized mice (Fig. 1).
Mucosal Lymphoid Changes in Rectal Tissues
Cytokine-Related Changes in Rectal Tissues
As detected by immunohistochemical staining using cytokine-specific antibodies, cryostat section from frozen tissue showed consecutive expression of the different cytokines with localization in the rectal mucosa which was different from that found in healthy controls (Table I). In general, the localization of intracellular expression of cytokines in infected mice was insignificantly lower than that seen in healthy controls. However, faint staining of the epithelial surface was observed for most of the cytokines. The IL-2 and IFN-γ expressions at the epithelial surface of the infected mice were absent though it could be detected in the lamina propria and muscularis mucosa (Fig. 2 B, J), whereas any significant changes in the expression of IL-18 and TNF-α were not seen in infected mice than observed in healthy controls (Fig. 2 Q, U compared with R, V, respectively), whose few epithelium crypt ends had been staining lightly. Predominant localization of IL-4- and IL-10-expressing cells in the surface epithelium, crypts, and various cell types in lamina propria were extensively observed (Fig. 2 F, N), while the expression of IL-4 and IL-10 was nearly absent in healthy controls (Fig. 2 E, M). Staining for such cytokines did not show any significant difference.
Intracellular localization of the different cytokines in cryopreserved rectal tissue sections obtained from control BALB/c mice (A, E, I, M, Q, U) after infection with a lethal dose of S. dysenteriae 1 (B, F, J, N, R, V), immunization with 57-kDa major antigenic fraction of the homologous strain (C, G, K, O, S, W) followed by infection (D, H, L, P, T, X) and from the control mice. Frozen tissue sections were stained by an immunofluorescent method using FITC-labeled anti-IL-2 (A–D), IFN-γ (I–L), and IL-18 (Q–T) or PE-labeled IL-4 (E–H), IL-10 (M–P), and TNF-α (U–X) and taken at a magnification of ×512
Mice infected with S. dysenteriae 1 expressed a low reactivity of cytokines in the rectal tissue in comparison to immunized and challenged–immunized mice following infection. A selective downmodulation of IL-2, IFN-γ, and TNF-α was observed in rectal mucosa of infected mice (P < 0.05) as shown in Fig. 2 B, J, V, while upregulation of IL-4, IL-10, and IL-18 is correlated with the disease. As compared to immunized mice, there was increased image signal for expression of IL-2 and IFN-γ in mucosal surface and crypts and in lamina propria cells in the challenged–immunized mice (Fig. 2 C, K compared with D, L). This level of IL-2- and IFN-γ-expressing cells in the lamina propria and crypts was also found to be statistically higher (P < 0.05) as compared with infected or healthy control mice (Table I). However, the numbers of IL-4- and IL-10-expressing cells in the epithelial surface or lamina propria were significantly lower at this level (Fig. 2 H, P compared with G, O) yet insignificant to that in infected mice (Fig. 2 H, P compared with F, N). There were no significant changes in the expression of the IL-18 and TNF-α as compared to immunized mice or healthy controls (Fig. 2 T, X compared with S, W or Q, U). Intracellular expression of IL-2 and IL-4 in the rectal tissues showed almost similar pattern found in the culture supernatant restimulated with 57-kDa antigenic fraction of immunized mice and following infection [32].
Expression of T Cell Markers in Rectal Tissues
Phenotypic expressions of CD4, CD8, and CD45 at the mucosal site were studied in the cryopreserved rectal tissues obtained from the healthy control mice, S. dysenteriae 1-infected mice, immunized, and infection after immunization with a lethal dose of bacteria. Figure 3 reveals that the ratio of CD4+/CD8+ cells out of the total CD3+ T cells (CD4/CD8+/CD3+; 2.62) was higher in the healthy controls as compared to Shigella-infected mice (CD4/CD8+/CD3+; 1.47). The expression level of CD45+ cells was observed to be lower in healthy control (CD45+; 11.83% ± 2.6) than the infected ones (CD45+; 13.36% ± 2.7). These levels were found to be significantly higher in immunized mice following infection (CD4/CD8/CD3+ = 3.11) than that of infected (CD4/CD8/CD3+ = 1.47; P < 0.02) mice (Fig. 3 H, L compared with G, K or F, J). These levels were found to be insignificant to immunized (CD4/CD8/CD3+ = 2.5) or healthy controls (CD4/CD8/CD3+ = 2.62); however, they were statistically higher at variance of mean value (Fig. 3 H, L compared with E, I). The expression of CD45+ T cells in challenged–immunized mice (CD45 = 29.19% ± 0.5) was more significant (P < 0.05) than that in infected or healthy control mice (Fig. 3 D compared with B or A) but insignificant to that in immunized mice (21.69% ± 1.3; Fig. 3 D compared with C).
Expression of CD4+, CD8+, and CD45+ in cryopreserved rectal tissues obtained from control BALB/c mice (A, E, I) from S. dysenteriae 1-infected (B, F, J), 57-kDa antigen-immunized (C, G, K), followed by infection with a lethal dose of homologous strain (D, H, L). E–L pictures showing after merging the two images CD3 and CD4 or CD3 and CD8. Blue fluorescence (white arrows) represents the expression of CD45+ T cells (A–D). Green fluorescence represents the positive expression of CD4+ (E–H) or CD8+ (I–L) out of CD3+ T cells taken at a magnification of ×107
Apoptosis During T Cell Differentiation in Rectal Tissues
Phenotype of the apoptotic cells was determined using differential immunostaining technique. Table II shows the percent expression of CD4+, CD8+, and CD45+ cells that underwent extensive apoptosis in infected mice as compared to healthy control or immunized mice. There were insignificant changes in the expression of CD4+, CD8+, and CD45+ apoptotic cells in immunized mice, but they were higher than in healthy controls. Later, the percentage of expression of CD8+ apoptotic T cells (25%) was observed to be significantly higher (P < 0.02) than CD4+ (10%) and CD45+ (4%) apoptotic T cells in immunized mice following Shigella challenge. On the other hand, annexin V-positive cells were highly expressed during infection and were found to be more significant (P < 0.05) to that in healthy controls (Fig. 4a, b). Although apoptotic cells for annexin V were decreased by three- to fourfold after 57-kDa antigen immunization, yet they were insignificant following infection (Fig. 4c, d). Such expressions in challenged–immunized mice were similar to the expression levels observed in healthy controls (Fig. 4a, d). Upregulation of apoptosis-related annexin V expression was predominantly on CD8+/CD3+.
Apoptosis in cryopreserved rectal tissue sections obtained from control BALB/c mice (a), S. dysenteriae 1-infected mice (b), immunized mice (c), followed by infection with a lethal dose of homologous strain (d). Bright yellow (black arrows) fluorescence indicates annexin V-positive cells localized in the lymphoid aggregate (magnification taken at ×107)
Caspase-1/Caspase-3 Activation During T Cell Differentiation
Expression of caspase-3 proteins was observed to be significantly higher in the rectal tissue of infected mice as compared to that in healthy controls (Fig. 5 A-II, E-II, compared with B-II, F-II, respectively), whereas caspase-1-expressing cells in infected mice were similar to the expression found in healthy control mice (Fig. 5 A-I, E-I, compared with B-I, F-I, respectively). Annexin V-positive caspase-3-expressing cells were observed in the epithelial surface, lamina propria, and lymphoid aggregates during Shigella challenge. These double positive cells were absent in the healthy controls. There was a marked change in activation of caspase-1 in immunized mice and infection postimmunization (Fig. 5 C-I, G-I, D-I, H-I), and the expression level of caspase-1 was found to increase (Table I), while such mice did not undergo any significant changes in activation of caspase-3 (Fig. 5 C-II, G-II, D-II, H-II). On the other hand, the expression of Bcl-2 in such infected mice was similar to that of healthy controls. However, a sharp increase in the expression of Bcl-2 was seen in the immunized mice following infection and was comparable to that in immunized or healthy control mice at 95% confidence limit as shown in Table I.
Immunohistochemical localization of apoptosis-related proteins (caspase-1 and caspase-3) in cryopreserved rectal tissues obtained from control BALB/c mice (A-I, E-I and A-II, E-II), S. dysenteriae 1-infected mice (B-I, F-I and B-II, F-II), immunized mice (C-I, G-I and C-II, G-II) and postimmunization infection with a lethal dose of homologous strain (D-I, H-I and D-II, H-II). The tissue sections were labeled with FAM-YVAD-FMK for caspase-1 and FAM-DEVD-FMK for caspase-3 for 1 h. Caspase activity (A-I–DI, AII–DII) was detected using a band pass filter (excitation 490 nm, emission 520 nm), and nuclear staining by Hoechst stain was observed using a UV filter (excitation 365 nm, emission 480 nm). To exclude the dead cells, these sections were further stained with propidium iodide (E–H) and taken at a magnification of ×512. Green fluorescence represents caspase-positive cells
Discussion
It is considered that most infectious diseases are initiated through invasion of mucosal tissues. Hence, mucosal immunity would be considered for the development of candidate vaccine. Experimental evidences suggest that pathogenesis due to Shigella involves both apoptosis and necrosis of the epithelial cells and the lamina propria cells [1, 11, 42]. S. flexneri is capable of inducing apoptosis in macrophages in vitro as well as in vivo [12, 14, 43, 44]. S. dysenteriae 1-infected patients are associated with extensive apoptotic cell death in the rectal mucosa, predominantly in CD3+ T cells infiltrating the lamina propria and lymphoid aggregates combined with downregulation of IL-2 and Bcl-2 [24]. Reactive oxygen species are the key mediators of necrotic cell death observed during acute shigellosis [45, 46]. In vitro studies have shown that IpaB of virulent Shigella spp. mediates the activation of caspase-1 and the cleavage of pro-inflammatory cytokines pro-IL-1β and pro-IL-18 into mature forms [13, 47, 48], resulting in inflammation [17]. Furthermore, pro-inflammatory cytokines such as IL-1β and IFN-γ are involved in antimicrobial defenses [49, 50] by inducing IL-18 [51, 52] to exert pleiotropic effect [16]. In the present study, an attempt was made to understand the mechanism underlying the lymphoid T cell responses following infection with Shigella before and after immunization. The effect of immunization triggers the signaling events affecting cellular differentiation. Earlier, EtxB has been shown to activate the cytokine secretion and caspase-1 during cellular differentiation inducing CD8+ T cell apoptosis [53]. We have investigated the processes by which 57-kDa antigen induces apoptosis in CD8+ T cell during cellular differentiation.
It was observed that CD4+ T cell expression increased in lamina propria and crypts of the 57-kDa immunized mice following infection and was significantly higher (P < 0.02) than that in infected mice (Fig. 3 H, L compared with G, K) with substantial increased expression of IL-2 and IFN-γ (Fig. 2 C, K compared with B, J). However, these levels of expressions were insignificant compared to immunized mice. Reduced expression of IL-2 and IFN-γ during infection (Table I) may have contributed to a lack of lymphocyte survival [31, 54]. Downregulation of IL-2 and IFN-γ during acute shigellosis was also reported earlier [55]. However, their increase at convalescent stage of day 30 strongly supported the hypothesis presented by Raqib et al. [21]. In a previous in vitro study, we have also suggested that exogenous anti-IFN-γ significantly induces IL-2 and IFN-γ levels in early stage of infection, which signifies the role of IFN-γ in antimicrobial host defense [55]. We have also observed that intracellular expression of IL-2 and IL-4 in the rectal tissues of the challenged–immunized mice showed a reciprocal relation as observed in our earlier in vitro experiments [32, 56]. Also, the levels of IFN-γ, IL-18, and TNF-α were found to be significantly elevated in such group to that of other comparable groups (Table I; Fig. 2 L, T, X compared with J, R, V or K, S, W, respectively).
On the other hand, a majority of annexin V-positive CD8 cells underwent apoptosis extensively in infected mice as compared to healthy controls (Table II). Apoptotic cells bearing CD8 subset marker in the lamina propria and lymphoid aggregates of the challenged–immunized mice suggested that an activation of selective Fas-mediated T cell death pathway was upregulated during infection following successful immunization. Furthermore, increased caspase-3 activity in the epithelial surface, lamina propria, and lymphoid aggregates during Shigella infection was observed (Fig. 5 A-II, E-II), whereas no significant changes were observed after immunization or following homologous infection indicating that activated caspase-3 did not affect the CD4 or CD8 cell apoptosis. Caspase-1-expressing cells in such challenged–immunized mice were greater with a decrease in caspase-3 activity (Table I). Data may suggest that caspase-3 is downregulated in CD4+ cells of the immunized BALB/c mice following infection while caspase-1 is upregulated in CD8+ T cells. It is assumed that the role of caspase-1 in inducing the CD4+ T helper cell activity increased with IL-18. It was seen to have persistent upregulated expression of IL-18, a substrate of caspase-1-producing cells in the rectal tissues of the challenged–immunized mice (Figs. 2 T and 4 D-I, H-I). IL-18 promotes inflammation and induces IFN-γ and TNF-α [40] as the expression of IFN-γ and TNF-α cytokines was evident in this study. Nevertheless, upregulated IL-18 perpetuated the inflammation with the level of TNF-α (Table I). The upregulation of IL-4, IL-10, and IL-18 is correlated with the severity of the disease [23, 30] and is observed to be significantly elevated (P < 0.05) during infection than those in healthy controls (Fig. 2 F, N, R compared with E, M, Q). These expressions were downregulated after successful immunization with an increased caspase-1 activity. It is known that caspase-1 is important in innate host defense against E. coli infection [57]. The findings that majority of the apoptotic cells were CD8+ T cells in challenged–immunized mice suggested that there might be a selective deletion of T lymphocytes mediated by caspase-1 via IL-18 in turn leading to CD4+ helper T cell activation with increased IL-2, IFN-γ, and TNF-α.
References
Sansonetti PJ. Molecular and cellular mechanisms of invasion of the intestinal barrier by enteric pathogens: the paradigm of Shigella. Folia Microbiol. 1998;43:239–46.
Clerc P, Baudry B, Sansonetti PJ. Molecular mechanism of entry, intracellular multiplication and killing of host cells by Shigellae. Curr Trop Microbiol Immunol. 1988;138:3–13.
Sansonetti PJ, Ryter A, Clerc P, Maurelli AT, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid mediated contact hemolysis. Infect Immun. 1986;51:461–9.
Bernardini M, Mounier L, D’Hauteville J, Coquis-Randon HM, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs intra- and inter-cellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–71.
Mathan MM, Mathan VI. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991;13 Suppl 4:S314–8.
Mathan MM, Mathan VI. Ultrastructural pathology of the rectal mucosa in Shigella dysentery. Am J Pathol. 1986;123:25–38.
Mizoguchi A, Mizoguchi E. Immune networks in animal models of inflammatory bowel diseases. Inflamm Bowel Dis. 2003;9:246–59.
Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infection: role in disease pathogenesis and host defense. Curr Opin in Microbiol. 1999;2:99–105.
DuPont HL, Pickering LK. Infections of the gastrointestinal tract. Microbiology, pathophysiology and clinical features. New York: Plenum; 1980. p. 73–4.
Islam D, Bardhan PK, Lindberg AA, Christeusson B. Shigella infection induces cellular activation of T and B cells and distinct species-related changes in peripheral blood lymphocyte subsets during the course of the disease. Infect Immun. 1995;63:2941–9.
Perdomo JJ, Cavaillon JM, Huerre M, Ohayon H, Gounon P, Sansonetti PJ. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–19.
Zychlinsky A, Fitting C, Cavaillon JM, Sansonetti PJ. Interleukin-1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Invest. 1994;94:1328–32.
Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, et al. Shigella-induced apoptosis is dependent on caspases-1, which binds to IpaB. J Biol Chem. 1998;273:32895–900.
Zychlinsky A, Kenny B, Menard R, Prevost MC, Holland IB, Sansonetti PJ. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–27.
Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J Clin Immunol. 1999;19:1–11.
Dinarello CA. Interleukin-18. Methods. 1999;19:121–32.
Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri induced inflammation. Immunity. 2000;12(5):581–90.
Louise CB, Obrig TG. Shiga toxin-associated hemolytic-uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1β and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect Immun. 1991;59:4173–9.
Simon HU. Regulation of eosinophil and neutrophil apoptosis—similarities and differences. Immunol Rev. 2001;179:156–62.
Murray J, Barbara JA, Dunkley SA, Lopez AF, Van Ostade X, Condliffe AM, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 induction of apoptosis in-vitro. Blood. 1997;90:2772–83.
Raqib R, Gustafsson A, Andersson J, Bakhiet M. A systemic downregulation of gamma interferon production is associated with acute shigellosis. Infect Immun. 1997;65:5338–41.
Raqib R, Ljungdahl A, Lindberg AA, Andersson U, Andersson J. Local entrapment of IFN-γ in the recovery from Shigella dysenteriae type 1 infection. Gut. 1996;38:328–36.
Raqib R, Lindberg AA, Bjork L, Bardhan PK, Wretlind B, Andersson U, et al. Down-regulation of gamma interferon, tumor necrosis factor type1, interleukin (IL-1) type 1, IL-3, IL-4 and transforming growth factor β type 1receptor at the local site during the acute phase of Shigella infection. Infect Immun. 1995;63:3079–87.
Raqib R, Ekberg C, Sharkar P, Bardhan PK, Zychlinsky A, Sansonetti PJ, et al. Apoptosis in acute shigellosis is associated with increase production of Fas/Fas ligand perforin, caspase-1 and caspase-3 but reduced production of Bcl-2 and interleukin-2. Infect Immun. 2002;70:3199–207.
Dinari G, Hale TL, Austin SW, Formal SB. Local and systemic antibody response to Shigella infection in Rhesus monkey. J Infect Dis. 1987;155:1065–9.
Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, et al. Prospective study of systemic and mucosal immune response in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59:2341–50.
Kärnell A, Stocker BAD, Katakura S, Reinholt FP, Lindberg AA. Live oral auxotrophic Shigella flexneri SFL124 vaccine with a deleted aroD gene: characterization and monkey protection studies. Vaccine. 1992;10:389–94.
Cleary TG, Winsor DK, Reich D, Ruiz-Palacios G, Calva JJ. Human milk immunoglobulin A antibodies to Shigella virulence determinants. Infect Immun. 1989;57:1675–9.
Cam PD, Pal T, Lindberg AA. Immune response against lipopolysaccharide and invasion plasmid coded antigens of Shigella flexneri in Vietnamese and Swedish dysenteric patients. J Clin Microbiol. 1993;31:454–7.
Raqib R, Wretlind B, Andersson J, Lindberg AA. Cytokine secretions in acute shigellosis in correlated to disease activity and directed more to stool than to plasma. J Infect Dis. 1995;171:376–84.
Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immune. 1995;63:289–96.
Bagchi AK, Sinha AK. Role of 57 kDa major antigenic component of Shigella dysenteriae outer membrane proteins in induction of major histocompatibility complex II-restricted T-cell response. Archiv Med Res. 2004;35:428–34.
World Health Organization. Manual for laboratory investigations of acute enteric infections. Publication CDD 83. Geneva: WHO; 1993. p. 3.
Sereny B. Experimenta kerato-conjunctivities shigellosis. Acta Microbiol Acad Sci Hung. 1957;4:367–76.
Johnston KH, Gotschlich EC. Isolation and characterization of the outer membrane of Neisseria gonorrhea. J Bacteriol. 1974;119:250–7.
Sinha AK, Chakraborti S, Chakraborti MK. Delayed hypersensitivity in relation to host cellular immune responses in Shigella infected mice. Immunol & Infect Dis. 1994;4:149–54.
Markwell MA, Hess SM, Beiber LL, Tolbert ME. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Annalyt Biochem. 1978;87:206–10.
Yin TE, Galanos C, Kinsky S, Bradshaw RA, Wessler S, Luderitz O, et al. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972;261:284–9.
Mallet CP, Hale TL, Kaminski RW, Larsen T, Orr N, Cohen D, et al. Intranasal or intragastric immunization with proteosomes-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–6.
Voller A, Bratlett A, Bidwell DE. Enzyme immunoassay with special reference to ELISA technique. J Clin Pathol. 1978;31:507–20.
Björk L, Andersson U, Chauvet JM, Skansen-Saphir U, Anderson J. Quantification of superantigens induced IFN-γ production by computerized image analysis—inhibition of cytokine production and blast cell formation by pooled human IgG. J Immunol Method. 1994;175:201–13.
Sansonetti PJ. Molecular and cellular biology of Shigella flexneri invasiveness: from cell assay system to shigellosis. Curr Trop Microbiol Immunol. 1992;180:1–19.
Zychlinsky A, Thirumalai K, Arondel J, Cantey JR, Aliprantis AO, Sansonetti PJ. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–65.
Zychlinsky AM, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9.
Raqib R, Mia SM, Qadri F, Alam TI, Alam NH, Chowdhury AK, et al. Innate immune responses in children and adults with shigellosis. Infect Immun. 2000;68:3620–9.
Wyllie AH. Apoptosis: an overview. Brit Med Bull. 1997;53:451–65.
Hilbi H. Host responses to secreted Shigella virulence factors. Curr Opinion Infects Dis. 1999;12:221–8.
Sansonetti PJ, Mournier J, Prevost MC, Mege RM. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 1994;76:829–39.
Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab Investig. 2000;80:759–67.
Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T-cell subsets. J Exp Med. 1989;169:59–72.
Eckmann L, Kagnoff MF. Cytokines in host defense against Salmonella. Microbes Infect. 2001;3:1191–200.
Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, et al. IL-18 (IFN-γ inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307.
Salmond RJ, Pitman RS, Jimi E, Soriani M, Hirst TR, Ghosh S, et al. CD8+ T cell apoptosis induced by Escherichia coli heat-labile enterotoxin B subunit occurs via a novel pathway involving NF-kappaB-dependent caspase activation. Eur J Immunol. 2002;32:1737–47.
Akber AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72–6.
Sinha AK, Bagchi AK. Cytokine release induced by killed bacteria associated with anti-IFN-γ antibody in Shigella infection. Cytokine. 2005;31:87–93.
Sinha AK, Bagchi AK. Role of anti-CD3 in modulation of Th1-type immune response in Shigella dysenteriae infection. J Med Microbiol. 2004;53:1075–81.
Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun. 2002;70:6896–903.
Acknowledgment
The authors are grateful to Mr. Sunayan Ghose, Division of Electron Microscopy, National Institute of Cholera & Enteric Diseases, Kolkata, for providing technical assistance for this work. Authors are also grateful to Dr. S. K. Bhattacharya, former Director, National Institute of Cholera & Enteric Diseases, Kolkata, India for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bagchi, A.K., Sinha, A.K., Adhikari, R. et al. Selective Deletion of CD8+ Cells Upregulated by Caspases-1 via IL-18 in Mice Immunized with Major Outer Membrane Protein of Shigella dysenteriae 1 Following Infection. J Clin Immunol 30, 408–418 (2010). https://doi.org/10.1007/s10875-009-9359-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-009-9359-8




 ) or postimmunized (
) or postimmunized ( ) BALB/c mice (five animals in each group of colony-forming unit dose)
) BALB/c mice (five animals in each group of colony-forming unit dose)


