Abstract
Background
Peripheral blood CD4+ and CD8+ T-cell subsets lacking surface CD28 have been suggested to predispose patients to immune-mediated disorders.
Materials and Methods
To determine the role of CD28− T-cell subset in Graves’ disease (GD), we characterized peripheral blood CD4+CD28− and CD8+CD28− T cell from early onset GD patients.
Results and Discussion
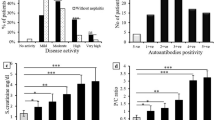
GD patients had significantly higher percentages of CD4+CD28− and CD8+CD28− T cells than did healthy donors. Both CD28− T cells expressed mostly CD45RO, suggesting that they are activated and/or are memory T cells. GD patient-derived CD4+CD28− and CD8+CD28− T cells produced more intracellular IFN-γ than their counterparts from healthy donors. Furthermore, CD4+CD28− and CD8+CD28− T cells from GD patients with Graves’ ophthalmopathy (GO) secreted higher level of intracellular IFN-γ than those CD28− T cells from GD patients without GO. Retrospective analysis showed that the increased levels of CD4+CD28− T cells and their IFN-γ-producing subgroups were positively correlated to the serum anti-thyrotropin receptor (TSHR) autoantibodies (TRAb). Our observations suggest that increased IFN-γ-producing CD28− T cells in GD patients may play an important role in the pathogenesis of GD.






Similar content being viewed by others
References
Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–717.
Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24:802–35.
McIver B, Morris JC. The pathogenesis of Graves’ disease. Endocrinol Metab Clin North Am. 1998;27:73–89.
Paschke R, Schuppert F, Taton M, Velu T. Intrathyroidal cytokine gene expression profiles in autoimmune thyroiditis. J Endocrinol. 1994;141:309–15.
Pichurin P, Yan XM, Farilla L, Guo J, Chazenbalk GD, Rapoport B, McLachlan SM. Naked TSH receptor DNA vaccination: a Th1 T cell response in which interferon-g production, rather than antibody, dominates the immune response in mice endocrinology. Endocrinology. 2001;142:3530–6.
Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99–107.
Dayan CM, Londei M, Corcoran AE, Grubeck-Loebenstein B, James RFL, Rapoport B, Feldmann M. Autoantigen recognition by thyroid-infiltrating T cells in Graves’ disease. Proc Natl Acad Sci U S A. 1991;88:7415–9.
Londei M, Bottazzo GF, Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985;228:85–9.
Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nature Rev Immunol. 2002;2:116–26.
Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–17.
Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77.
Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J Biol Chem. 1998;273:8119–29.
Choremi-Papadopoulou H, Viglis V, Gargalianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J Acquir Immune Defic Syndr. 1994;7:245–53.
Schmidt D, Goronzy JJ, Weyand CM. CD4+CD7−CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–37.
Duftner C, Goldberger C, Falkenbach A, Würzner R, Falkensammer BP, Feiffer KP, et al. Prevalence, clinical relevance and characterization of circulating cytotoxic CD4+CD28− T cells in ankylosing spondylitis. Arthritis Res Ther. 2003;5:292–300.
Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R. CD4+CD28− costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–94.
Lamprecht P, Moosig F, Csernok E, Seitzer U, Schnabel A, Mueller A, et al. CD28 negative T cells are enriched in granulomatous lesions of the respiratory tract in Wegener’s granulomatosis. Thorax. 2001;56:751–7.
Liuzzo G, Kopecky SL, Frye RL, Fallon WM, Maseri A, Goronzy JJ, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–9.
Namekawa T, Snyder MR, Yen JH, Goehring BE, Leibson PJ, Weyand CM, et al. Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J Immunol. 2000;165:1138–45.
Speiser DE, Valmori D, Rimoldi D, Pittet MJ, Lienard D, Cerundolo V, et al. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999;29:1990–9.
Giscombe R, Nityanand S, Lewin N, Grunewald J, Lefvert AK. Expanded T cell populations in patients with Wegener’s granulomatosis: characteristics and correlates with disease activity. J Clin Immunol. 1998;18:404–13.
Komocsi A, Lamprech P, Csernok E, Mueller A, Holl-Ulrich K, Seitzer U, et al. Peripheral blood and granuloma CD4+CD28− T cells are a major source of interferon-g and tumor necrosis factor-a in Wegener’s granulomatosis. Am J Pathol. 2002;160:1717–24.
Leeuwen MM, Remmerswaal BM, Vossen TM, Rowshani T, Wertheim-Dillen ME, Lier AW, et al. Emergence of a CD4+CD28− granzymeB+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalo virus infection. J Jmmunol. 2004;173:1834–41.
Matsuoka N, Eguchi K, Kawakami A, Tsuboi M, Nakamura H, Kimura H, et al. Lack of B7-1/BB1 and B7-2/B70 expression on thyrocytes of patients with Graves’ disease. Delivery of costimulatory signals from bystander professional antigen-presenting cells. J Clin Endocrinol Metab. 1996;81:4137–43.
Salvi M, Spaggiari E, Neri F, Macaluso C, Gardini E, Ferrozzi F, et al. The study of visual evoked potentials in patients with thyroid associated ophthalmopathy identifies asymptomatic optic nerve involvement. J Clin Endocrinol Metab. 1997;82:1027–30.
Mascher B, Schlenke P, Seyfarth M. Expression and kinetics of cytokines determined by intracellular staining using flow cytometry. J Immunol Methods. 1999;223:115–21.
Nociari MM, Telford W, Russo C. Development of CD28−CD8+ T cell subset: age-associated expansion and shift from memory to naïve phenotype. J Immunol. 1999;162:3327–35.
Vallejo AN, Brandes JC, Weyand CM, Goronzy JJ. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–9.
Park W, Weyand CM, Schmidt D, Goronzy JJ. Co-stimulatory pathways controlling activation and peripheral tolerance of human CD4+CD28− T cells. Eur J Immunol. 1997;27:1082–90.
Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-a. J Immunol. 2001;167:3231–8.
Sprent J. Immunological memory. Cur Opin Immunol. 1997;9:371–9.
Dubey C, Croft M, Swain L. Naïve and effector CD4+ T cells differ in their requirements for T cells receptor versus costimulatory signals. J Immunol. 1996;157:3280–9.
Duftner C, Seiler R, Klein-Weigel WP, Göbel H, Goldberger C, Ihling C, et al. High prevalence of circulating CD4+CD28− T-cells in patients with small abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2005;25:1347–52.
Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100:550–7.
Pichurin P, Pichurina O, Chazenbalk GD, Paras C, Chen CR, Rapoport B, et al. Immune deviation away from Th1 in interferon-g knock-out mice does not enhance TSH receptor antibody production following naked DNA vaccination. Endocrinology. 2002;143:1182–9.
Nagayama Y, Mizuguchi H, Hayakawa T, Niwa M, McLachlan SM, Rapoport B. Prevention of autoantibody-mediated Graves’-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol. 2003;170:3522–7.
Kumar S, Bahn RS. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2003;88:4246–4250.
Karasek M, Lewinski A. Etiopathogenesis of Graves’ disease. Neuro Endocrinol Lett. 2003;24:161–6.
McLachlan SM, Nagayama Y, Rapoport B. Insight into Graves’ hyperthyroidism from animal models. Endocr Rev. 2005;26:800–32.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program of China (2001 CB51003) and the National Natural Science Foundation of China (30471690). We thank Prof. Andrej and Dr. Yi Zhang for critical reading of the manuscript. Ge-Hua Yu is thanked for excellent technical assistance. We also thank Dr. Shi and Dr. Chen for their help with the collection of peripheral blood samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhiping Sun and Weixue Zhong contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Sun, Z., Zhong, W., Lu, X. et al. Association of Graves’ Disease and Prevalence of Circulating IFN-γ-producing CD28− T Cells. J Clin Immunol 28, 464–472 (2008). https://doi.org/10.1007/s10875-008-9213-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9213-4