Abstract
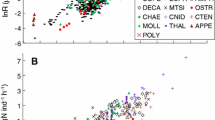
Respiration and ammonia excretion data and chemical composition data [water content, ash, carbon (C), nitrogen (N) and C:N ratios] of 18–32 amphipods (hyperiids and gammarids) from the epipelagic through bathypelagic zones of the world’s oceans were compiled. The independent variables including body mass, habitat temperature and mid-sampling depth were all significant predictors of respiration, accounting for 65–83 % of the variance in the data, while the former two variables were significant predictors of ammonia excretion, accounting for 64–77 % of the variance. Atomic O:N ratios (respiration:ammonia excretion) ranged from 11 to 74 (median 21.5). C composition was negatively correlated with habitat temperature, but water contents, ash, N, and the C:N ratio were uncorrelated with the three independent variables. As judged by C:N ratios, protein was considered to be the major organic component of most pelagic amphipods. However, some amphipods from >500 m depth exhibited high C:N ratios (>10) suggesting a large deposition of lipids in the body. Comparison of the present results with global bathymetric models of euphausiids and pelagic copepods revealed that respiration rates of the pelagic amphipods were near-equal to the rates of euphausiids but greater than the rates of pelagic copepods, reflecting taxon-specific body morphology and swimming behavior among the three taxa. As a marked feature of body chemical composition, the pelagic amphipods exhibited extremely high ash content (mean 25 % of DM) due to their possession of a robust exoskeleton.





Similar content being viewed by others
References
Båmstedt U (1986) Chemical composition and energy content. In: Corner EDS, O’Hara SCM (eds) The biological chemistry of marine copepods. Clarendon, Oxford, pp 1–58
Bocher P, Cherel Y, Labat JP, Mayzaud P, Razouls S, Jouventin P (2001) Amphipod-based food web: Themisto gaudichaudii caught in nets and by seabirds in Kerguelen waters, southern Indian Ocean. Mar Ecol Prog Ser 223:261–276
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Red Bd Can 21:1184–1226
Buskey EJ (1998) Energetic costs of swarming behaviour for the copepod Dioithona oculata. Mar Biol 130:425–431
Childress JJ (1975) The respiratory rates of midwater crustaceans as a function of depth occurrence and relation to the oxygen minimum layer off Southern California. Comp Biochem Physiol A 50:787–799
Childress JJ (1995) Are there physiological and biochemical adaptation of metabolism in deep-sea animals? Trends Ecol Evol 10:30–36
Childress JJ, Nygaard MH (1974) The chemical composition and relative buoyancy of midwater crustaceans as a function of depth off southern California. Mar Biol 27:225–238
Clarke A (1987) The adaptation of aquatic animals to low temperatures. In: Grout BWW, Morris GJ (eds) The effects of low temperatures on biological systems. Edward Arnold, London, pp 315–348
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Conover RJ (1960) The feeding behavior and respiration of some marine planktonic Crustacea. Biol Bull 119:399–415
Conover RJ, Corner EDS (1968) Respiration and nitrogen excretion by some marine zooplankton in relation to their life cycles. J Mar Biol Assoc UK 48:49–75
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Halcrow K, Boyd CM (1967) The oxygen consumption and swimming activity of the amphipod Gammarus oceanicus at different temperatures. Comp Biochem Physiol 23:233–242
Haro-Garay MJ (2004) Diet and functional morphology of the mandible of two planktonic amphipods from the Strait of Georgia, British Columbia, Parathemisto pacifica (Stebbing, 1988) and Cyphocaris challenger (Stebbing, 1988). Crustaceana 76:1291–1312
Haro-Garay MJ (2008) Dominance shift of zooplankton species composition in the central Strait of Georgia, British Columbia during 1997. Hidrobiológica 18:53–60
Hiller-Adams P, Childress JJ (1983) Effects of feeding, feeding history, and food deprivation on respiration and excretion rates of the bathypelagic mysid Gnathophausia ingens. Biol Bull 165:182–196
Hirche H-J (1984) Temperature and metabolism of plankton-I. Respiration of Antarctic zooplankton at different temperatures with a comparison of Antarctic and Nordic krill. Comp Biochem Physiol 77A:361–368
Hopkins TL (1985) Food web of an Antarctic midwater ecosystem. Mar Biol 89:197–212
Ikeda T (1974) Nutritional ecology of marine zooplankton. Mem Fac Fish Hokkaido Univ 22:1–97
Ikeda T (1977) The effect of laboratory conditions on the extrapolation of experimental measurements to the ecology of marine zooplankton. IV. Changes in respiration and excretion rates of boreal zooplankton species maintained under fed and starved conditions. Mar Biol 41:241–252
Ikeda T (1985) Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Mar Biol 85:1–11
Ikeda T (1988) Metabolism and chemical composition of crustaceans from the Antarctic mesopelagic zone. Deep-Sea Res 35:1991–2002
Ikeda T (1991) Assimilated carbon budget for the hyperiid amphipod Themisto japonica (Bovallius) from the Japan Sea as influenced by temperature. J Oceanogr Soc Jpn 47:94–103
Ikeda T (2012) Metabolism and chemical composition of zooplankton from 500 to 5,000 m depth of the western subarctic Pacific Ocean. J Oceanogr 68:641–649
Ikeda T (2013) Respiration and ammonia excretion of euphausiid crustaceans: synthesis towards a global-bathymetric model. Mar Biol 160:251–262
Ikeda T, Dixon P (1984) The influence of feeding on the metabolic activity of Antarctic krill (Euphausia superba Dana). Polar Biol 3:1–9
Ikeda T, Hirakawa K (1998) Metabolism and body composition of zooplankton in the cold mesopelagic zone of the southern Japan Sea. Plankton Biol Ecol 45:31–44
Ikeda T, Mckinnon DA (2012) Metabolism and chemical composition of zooplankton and hyperbenthos from the Great Barrier Reef waters, North Queensland, Australia. Plankton Benthos Res 7:8–19
Ikeda T, Mitchell AW (1982) Oxygen uptake, ammonia excretion, and phosphate excretion of krill and other Antarctic zooplankton, in relation to their body size and chemical composition. Mar Biol 71:283–298
Ikeda T, Shiga N (1999) Production, metabolism and production/biomass (P/B) ratio of Themisto japonica (Crustacea: Amphipoda) in Toyama Bay, southern Japan Sea. J Plankton Res 21:299–308
Ikeda T, Skjoldal HR (1989) Metabolism and elemental composition of zooplankton from the Barents Sea during early arctic summer. Mar Biol 100:173–183
Ikeda T, Takahashi T (2012) Synthesis towards a global-bathymetric model of metabolism and chemical composition of marine pelagic chaetognaths. J Exp Mar Biol Ecol 424–425:78–88
Ikeda T, Torres JJ, Hernández-León S, Geiger SP (2000) Metabolism. In: Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M (eds) ICES zooplankton methodology manual. Academic, San Diego, pp 455–532
Ikeda T, Yamaguchi A, Matsuishi T (2006a) Chemical composition and energy content of deep-sea calanoid copepods in the western North Pacific Ocean. Deep Sea Res I 53:1791–1809
Ikeda T, Sano F, Yamaguchi A, Matsuishi T (2006b) Metabolism of mesopelagic and bathypelagic copepods in the western North Pacific Ocean. Mar Ecol Prog Ser 322:199–211
Ikeda T, Sano F, Yamaguchi A (2007) Respiration in marine pelagic copepods: a global-bathymetric model. Mar Ecol Prog Ser 339:215–219
Ivleva IV (1980) The dependence of crustacean respiration rate on body mass and habitat temperature. Int Rev Ges Hydrobiol 65:1–47
James MR, Wilkinson VH (1988) Biomass, carbon ingestion, and ammonia excretion by zooplankton associated with an upwelling plume in western Cook Strait, New Zealand. NZ J Mar Freshw Res 22:249–257
King FD, Packard TT (1975) Respiration and the activity of the respiratory electron transport system in marine zooplankton. Limnol Oceanogr 20:849–853
Krapp RH, Berge J, Flores H, Gulliksen B, Werner I (2008) Sympagic occurrence of Eusirid and Lysianassoid amphipods under Antarctic pack ice. Deep-Sea Res II 55:1015–1023
Kruse S, Brey T, Bathmann U (2010) Role of midwater chaetognaths in Southern Ocean pelagic energy flow. Mar Ecol Prog Ser 416:105–113
Laval P (1980) Hyperiid amphipods as crustacean parasitoids associated with gelatinous zooplankton. Oceanogr Mar Biol Annu Rev 18:11–56
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306
Longhurst AR (1985) The structure and evolution of plankton communities. Prog Oceanogr 15:1–35
Mayzaud P, Conover RJ (1988) O:N atomic ratio as a tool to describe zooplankton metabolism. Mar Ecol Prog Ser 45:289–302
Omori M, Ikeda T (1984) Methods in marine zooplankton ecology. Wiley, USA, p 332
Opalinski KW, Jażdżewski K (1978) Respiration of some Antarctic amphipods. Pol Arch Hydrobiol 25:643–655
Owens TG, King FD (1975) The measurement of respiratory electron-transport system activity in marine zooplankton. Mar Biol 30:27–36
Pakhomov EA, Perissinotto R (1996) Trophodynamics of the hyperiid amphipod Themisto gaudichaudii in the South Georgia region during late austral summer. Mar Ecol Prog Ser 134:91–100
Percy JA (1993) Energy consumption and metabolism during starvation in the Arctic hyperiid amphipod Themisto libellula Mandt. Polar Biol 13:549–555
Postel L, Fock H, Hagen W (2000) Biomass and abundance. In: Harris RP, Wiebe PH, Lenz J, Skjoldal HR, Huntley M (eds) ICES zooplankton methodology manual. Academic, San Diego, pp 83–192
Prosser CL (1961) Oxygen: respiration and metabolism. In: Prosser CL, Brown FA (eds) Comparative animal physiology. Saunders, Philadelphia, pp 165–211
Quetin LB, Ross RM, Uchio K (1980) Metabolic characteristics of midwater zooplankton: ammonia excretion, O:N ratios, and the effect of starvation. Mar Biol 59:201–209
Raymont JEG (1983) Plankton and productivity in the oceans: zooplankton, vol 2. Pergamon, Oxford
Schneppenheim R, Weigmann-Haass R (1986) Morphological and electrophoretic studies of the Genus Themisto (Amphipoda: Hyperiidea) from the south and North Atlantic. Polar Biol 6:215–225
Scholander PF, Flagg W, Walters V, Irving L (1953) Climatic adaptation in arctic and tropical poikilotherms. Physiol Zool 26:67–92
Seibel BA, Drazen JC (2007) The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Philos Trans R Soc Lond B 362:2061–2078
Sheader M, Evans F (1975) Feeding and gut structure of Parathemisto gaudichaudi (Guerin) (Amphipoda, Hyperiidea). J Mar Biol Assoc UK 55:641–656
Shih CT (1982) Hyperiidea. In: Parker SP (ed) Synopsis and classification of living organisms, 2nd edn. McGraw-Hill, New York, pp 285–292
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research. Freeman, New York
Torres JJ, Childress JJ (1983) Relationship of oxygen consumption to swimming speed in Euphausia pacifica. 1. Effects of temperature and pressure. Mar Biol 74:79–86
Torres JJ, Aarset AV, Donnelly J, Hopkins TL, Lancraft TM, Ainley DJ (1994a) Metabolism of Antarctic micronektonic Crustacea as a function of depth of occurrence and season. Mar Ecol Prog Ser 113:207–219
Torres JJ, Donnelly J, Hopkins TL, Lancraft TM, Aaset AV, Ainley DJ (1994b) Proximate composition and overwintering strategies of Antarctic micronektonic Crustacea. Mar Ecol Prog Ser 113:221–232
Ventura M (2006) Linking biochemical and elemental composition in freshwater and marine crustacean zooplankton. Mar Ecol Prog Ser 327:233–246
Vinogradov ME (1968) Vertical distribution of oceanic zooplankton. Israel Program for Scientific Translations, Jerusalem
Vinogradov ME, Volkov AF, Semenova TN (1982) Hyperiid amphipods (Amphipoda, Crustacea) of the Ocean. Leningrad: Nauka, p 492 (in Russian. English transl.: 1996, Smithsonian Institution Libraries, Washington, D.C.)
Yamada Y, Ikeda T (2003) Metabolism and chemical composition of four pelagic amphipods in the Oyashio region, western subarctic Pacific. Mar Ecol Prog Ser 253:233–241
Zeuthen E (1947) Body size and metabolic rate in the Animal Kingdom with special regard to the marine microfauna. CR Trav Lab Carlsberg (Sér chim) 26:17–161
Acknowledgments
I am grateful to two anonymous referees for their comments which improved the text. I thank D.A. McKinnon for his critical reading of early drafts of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikeda, T. Metabolism and chemical composition of marine pelagic amphipods: synthesis toward a global bathymetric model. J Oceanogr 69, 339–355 (2013). https://doi.org/10.1007/s10872-013-0177-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-013-0177-5