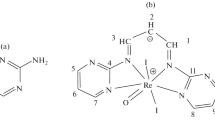
Abstract
The synthesis and crystal structures of 48 new rare-earth (RE = La3+—Y3+)-3,5-dihalogenated benzoic acid (3,5-dibromobenzoic acid [3,5-dBrBA] and 3-bromo-5-iodobenzoic acid [3,5-BrIBA])-terpyridine [TPY] complexes are reported. Ligand based supramolecular assembly drives the formation of five distinct structure types across the lanthanide series. Featured in these structures are multiple significant halogen bonding interactions occurring at the terminal halide substituents in the form of halogen–halogen, halogen–oxygen, and halogen–π interactions. This series complements previous efforts to synthesize and evaluate a catalogue of Ln-halobenzoic acid-TPY materials. With these data, a comparison of the influence of halogen interactions on supramolecular assembly is provided. As one might expect, the frequency with which halogen bonding occurs and the displacement of other assembly mechanisms, depends on the number and polarizability of the halogen species. Structures across these series fall into multiple distinct structure types, as defined by tecton geometry. Since these tectons are isostructural across each respective series, trends in halogen bond propensity can be derived. In this comparison, the likelihood of halogen bonding and disruption of π–π stacking is shown to increase as halogen size increases.
Graphic Abstract
Two new series of rare-earth (RE = La3+–Y3+)-3,5-dihalogenated benzoic acid (3,5-dibromobenzoic acid and 3-bromo-5-iodobenzoic acid)-terpyridine complexes are synthesized and compared, with respect to supramolecular assembly, to each other and previously reported RE-halogenated benzoic acid-terpyridine series in order to study the role of halogen bonding in this system.


















Similar content being viewed by others
References
Zhou B, Shi B, Jin D, Liu X (2015) Controlling upconversion nanocrystals for emerging applications. Nat Nanotechnol 10:924
Carter KP, Pope SJA, Cahill CL (2014) A series of Ln-p-chlorobenzoic acid–terpyridine complexes: lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm 16(10) (Part I)
Batrice RJ, Ridenour JA, Ayscue Ill RL, Bertke JA, Knope KE (2017) Synthesis, structure, and photoluminescent behaviour of molecular lanthanide–2-thiophenecarboxylate–2,2′:6′,2′′-terpyridine materials. CrystEngComm 19(35):5300–5312
Carter KP, Kalaj M, Cahill CL (2017) Harnessing uranyl oxo atoms via halogen bonding interactions in molecular uranyl materials featuring 2,5-diiodobenzoic acid and N-donor capping ligands. Inorg Chem Front 4(1):65–78
Gilday LC, Robinson SW, Barendt TA, Langton MJ, Mullaney BR, Beer PD (2015) Halogen Bonding in Supramolecular Chemistry. Chem Rev 115(15):7118–7195
Mukherjee A, Tothadi S, Desiraju GR (2014) Halogen bonds in crystal engineering: like hydrogen bonds yet different. Acc Chem Res 47(8):2514–2524
Ridenour JA, Carter KP, Butcher RJ, Cahill CL (2017) RE-p-halobenzoic acid–terpyridine complexes, Part II: structural diversity, supramolecular assembly, and luminescence properties in a series of p-bromobenzoic acid rare-earth hybrid materials. CrystEngComm 19(8):1172–1189 (Part II)
August Ridenour J, Carter KP, Cahill CL (2017) RE-p-halobenzoic acid–terpyridine complexes, part III: structural and supramolecular trends in a series of p-iodobenzoic acid rare-earth hybrid materials. CrystEngComm 19(8):1190–1203 (Part III)
Carter KP, Thomas KE, Pope SJA, Holmberg RJ, Butcher RJ, Murugesu M, Cahill CL (2016) Supramolecular assembly of molecular rare-earth-3,5-dichlorobenzoic acid-2,2':6',2''-terpyridine materials: structural systematics, luminescence properties, and magnetic behavior. Inorg Chem 55(14):6902–15 (Part IV)
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64(1):112–122
Hubschle CB, Sheldrick GM, Dittrich B (2011) ShelXle: a Qt graphical user interface for SHELXL. J Appl Crystallogr 44(6):1281–1284
Janiak C (2000) A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands†. J Chem Soc, Dalton Trans 21:3885–3896
Schollmeyer D, Shishkin OV, Ruhl T, Vysotsky MO (2008) OH–π and halogen–π interactions as driving forces in the crystal organisations of tri-bromo and tri-iodo trityl alcohols. CrystEngComm 10:715–723
Cotton S (2006) Lanthanide and actinide chemistry. Wiley, West Sussex
Acknowledgements
This work was supported internally by the Chemistry Department and the Columbian College of Arts and Sciences at The George Washington University. We would like to acknowledge the staff and instructors of American Crystallographic Association’s Summer Course in Chemical Crystallography (Summer 2019, Northwestern University, Evanston IL) for assistance in data collection and structure determination for select complexes presented in this paper. Finally, the authors are indebted to Mr. Gareth Nicholas (Haverford College) for synthesis efforts during a summer internship at GW.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Availability of Data
Unit cell parameters, data quality statistics and CCDC reference codes of all crystal structures are included in the electronic supplementary material.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herder, J.A., Walusiak, B.W. & Cahill, C.L. RE-Halobenzoic Acid-Terpyridine Complexes, Part V: Synthesis and Supramolecular Assembly of Rare-Earth-3,5-Dihalobenzoic Acid-Terpyridine Materials and Subsequent Comparison of Non-covalent Interactions. J Chem Crystallogr 51, 317–336 (2021). https://doi.org/10.1007/s10870-020-00858-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-020-00858-x