Abstract
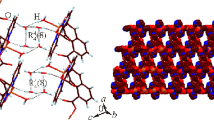
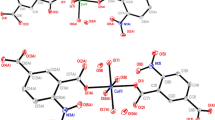
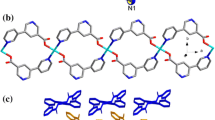
Three complexes: {[Mn(H2O)(mal)(5dmb)·H2O}n] (1); [Ni2(H2O)6(mal)2(4dmb)2]·3H2O (2); [Cu2(mal)2(4dmb)2]·3H2O (3); where mal = maleato, 4dmb = 4,4′-dimethyl-2,2′-bipyridine, and 5dmb = 5,5′-dimethyl-2,2′-bipyridine; have been synthesized, using self-assembly solution reactions at ambient conditions. Crystallographic studies show that 1 crystallizes in an orthorhombic system, space group Pna21, with a = 17.4067(4) Å, b = 11.9672(2) Å, c = 8.2075(2) Å; V = 1709.70(6) Å3. Complex 2 has a monoclinic system, space group C2/c, with a = 21.206(8) Å, b = 7.523(3) Å, c = 25.399(10) Å; β = 109.755(8)°; V = 3813(2) Å3. Complex 3 crystallizes in a monoclinic system, space group C2/c, with a = 14.6976(12) Å, b = 11.3849(10) Å, c = 22.1638(18) Å; β = 101.2998(17)°; V = 3636.8(5) Å3. Complex 1 is a one-dimensional (1D) polymer, where the Mn centers are six-coordinated in a distorted octahedral geometry. 2 is a dinuclear complex, generated by supramolecular interactions, where Ni ions are six-coordinated in a distorted octahedral geometry. 3 is a dinuclear complex with five-coordinated Cu ions having a distorted square pyramidal geometry. All three complexes exhibit hydrogen bonding interactions, which generate 2D supramolecular structures in 1 and 2, whereas in complex 3 a 3D supramolecular array is formed. These novel complexes prove that the self-assembly of a dicarboxylate ligand (mal) with three different first-row transition metals, can afford coordination compounds with diverse structural characteristics and dimensionality, which can be attributed to the different ligand coordination modes and the coordination properties of the employed metals.
Graphical Abstract
Divergent coordination compounds of three different transition metals have been obtained due to the versatility in the coordination modes of maleato ligand.








Similar content being viewed by others
References
Zhou HC, Long JR, Yaghi OM (2012) Chem Rev 112:673
Dua M, Li CP, Liub CS, Fang SM (2013) Coord Chem Rev 257:1282
Das D, Banerjee R, Mondal R,. Howard JAK, Boese R, Desiraju GR (2006) Chem Commun 555
Zhou XH, Li L, Li HH, Li A, Yang T, Huang W (2013) Dalton Trans 42:12403
Lusby PJ (2013) Annu Rep Prog Chem A 109:254
Lescop C (2017) Acc Chem Res 50(4):885
Ye BH, Tong ML, Chen XM (2005) Coord Chem Rev 249:545
Curiel D, Más-Montoya M, Sánchez G (2014) Coord Chem Rev 284:19
Rosales-Vázquez LD, Sánchez-Mendieta V, Dorazco-González A, Martínez-Otero D, García-Orozco I, Morales-Luckie RA, Jaramillo-García J, Téllez-López A (2017) Dalton Trans 46:12516
Téllez-López A, Jaramillo-García J, Martínez-Domínguez R, Morales-Luckie RA, Camacho-López MA, Escudero R, Sánchez-Mendieta V (2015) Polyhedron 100:373
Téllez-López A, Sánchez-Mendieta V, Jaramillo-García J, Rosales-Vázquez LD, García-Orozco I, Morales-Luckie RA, Escudero R, Morales-Leal F (2016) Trans Met Chem 41:879
Zhao RL, Yue KF, Zhou C, Cheng QDM, Shi JT, Liu YL, Wanga YY (2013) Inorg Chim Acta 402:25
Farnum GA, Martin DP, Sposato LK, Supkowski RM, LaDuca RL (2010) Inorg Chim Acta 363:250
Hancock RD (2013) Chem Soc Rev 42:1500
Alizadeh R, Amani V (2016) Inorg Chim Acta 443:151
Lopes LB, Corrêa CC, Guedes GP, Vaz MGF, Diniz R, Machado FC (2013) Polyhedron 50:16
Zhang GM, Li Y, Zou XZ, Zhang JA, Gu JZ, Kirillov AM (2016) Trans Met Chem 41:153
APEX 2 Software Suite. Bruker AXS Inc., Madison
Sheldrick GM (2008) Acta Crystallogr A 64:112
Hübschle CB, Sheldrick GM, Dittrich B, shelXle (2011) Appl Cryst 44:1281
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) Mercury CSD 2.0-New features for the visualization and investigation of crystal structures. J Appl Cryst 41:466
Rodríguez-Martín Y, Hernández-Molina M, Delgado FS, Pasán J, Ruiz-Pérez C, Sanchiz J, Lloret F, Julve M (2002) Cryst Eng Comm 4(87):522
Rodríguez-Martín Y, Hernández-Molina M, Delgado FS, Pasán J, Ruiz-Pérez C, Sanchiz J, Lloret F, Julve M (2003) Dalton Trans 11:2359
Ruiz-Pérez C, Hernández-Molina M, Sanchiz J, López T, Lloret F, Julve M (2000) Inorg Chim Acta 298:245
Jiang CH, Qi YM, Sun Y, Chi Q, Guo YM (2012) J Mol Struct 1017:65
Choudhury SR, Lee HM, Hsiao TH, Colacio E, Jana AD, Mukhopadhyay S (2010) J Mol Struct 967:131
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC (1984) J Chem Soc Dalton Trans 1349
Youngme S, Cheansirisomboon A, Danvirutai C, Pakawatchai C, Chaichit N (2008) Inorg Chem Commun 11:57
Tokii T, Watanabe N, Nakashima M, Muto Y, Morooka M, Ohba S, Saito Y (1990) Bull Chem Soc Jpn 63:364
Boonmak J, Youngme S, Chotkhun T, Engkagul C, Chaichit N, van Albada GA, Reedijk J (2008) Inorg Chem Commun 11:1231
Nath JK, Mondal A, Powell AK, Baruah JB (2014) Cryst Growth Des 14:4735
Das K, Panda U, Datta A, Roy S, Mondal S, Massera C, Askun T, Celikboyun P, Garribba E, Sinha C, Anand K, Akitsu T, Kobayashi K (2015) New J Chem 39:7309
Novoa N, Roisnel T, Dorcet V, Cador O, Manzur C, Carrillo D, Hamon JR (2016) New J Chem 40:5920
Mahapatra P, Ghosh S, Giri S, Rane V, Kadam R, Drew MGB, Ghosh A (2017) Inorg Chem 56:5105
Acknowledgements
Authors are thankful to M. en C. Alejandra Nuñez Pineda (CCIQS UAEM-UNAM) for elemental analysis of compounds. Funding for this work was provided by Universidad Autónoma del Estado de México.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Morales-Morales, N., Rodríguez-Olivas, M., Téllez-López, A. et al. Syntheses and Crystal Structures of Mn(II), Ni(II) and Cu(II) Coordination Compounds Assembled by Maleato and Dimethyl-2,2′-bipyridines. J Chem Crystallogr 49, 8–20 (2019). https://doi.org/10.1007/s10870-018-0731-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0731-5