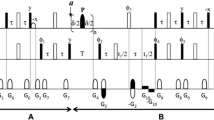
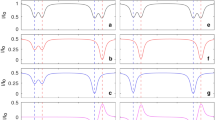
Abstract
Chemical Exchange Saturation Transfer (CEST) experiments are increasingly used to study slow timescale exchange processes in biomolecules. Although 15N- and 13C-CEST have been the approaches of choice, the development of spin state selective 1H-CEST pulse sequences that separate the effects of chemical and dipolar exchange [T. Yuwen, A. Sekhar and L. E. Kay, Angew Chem Int Ed Engl 2016 doi: 10.1002/anie.201610759 (Yuwen et al. 2017)] significantly increases the utility of 1H-based experiments. Pulse schemes have been described previously for studies of highly deuterated proteins. We present here longitudinal-relaxation optimized amide 1H-CEST experiments for probing chemical exchange in protonated proteins. Applications involving a pair of proteins are presented establishing that accurate 1H chemical shifts of sparsely populated conformers can be obtained from simple analyses of 1H-CEST profiles. A discussion of the inherent differences between 15N-/13C- and 1H-CEST experiments is presented, leading to an optimal strategy for recording 1H-CEST experiments.








Similar content being viewed by others
References
Anthis NJ, Clore GM (2015) Visualizing transient dark states by NMR spectroscopy. Q Rev Biophys 48:35–116. doi:10.1017/S0033583514000122
Banci L, Bertini I, Cramaro F, Del Conte R, Viezzoli MS (2002) The solution structure of reduced dimeric copper zinc superoxide dismutase. The structural effects of dimerization. Eur J Biochem 269:1905–1915. doi:10.1046/j.1432-1033.2002.02840.x
Bouvignies G, Kay LE (2012a) A 2D 13C-CEST experiment for studying slowly exchanging protein systems using methyl probes: an application to protein folding. J Biomol NMR 53:303–310. doi:10.1007/s10858-012-9640-7
Bouvignies G, Kay LE (2012b) Measurement of proton chemical shifts in invisible states of slowly exchanging protein systems by chemical exchange saturation transfer. J Phys Chem B 116:14311–14317. doi:10.1021/jp311109u
Bouvignies G, Vallurupalli P, Kay LE (2014) Visualizing side chains of invisible protein conformers by solution NMR. J Mol Biol 426:763–774. doi:10.1016/j.jmb.2013.10.041
Carr HY, Purcell EM (1954) Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev 94:630–638. doi:10.1103/PhysRev.94.630
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on Unix pipes. J Biomol NMR 6:277–293. doi:10.1007/Bf00197809
Deverell C, Morgan RE, Strange JH (1970) Studies of chemical exchange by nuclear magnetic relaxation in rotating frame. Mol Phys 18:553–559. doi:10.1080/00268977000100611
Farrow NA, Zhang OW, Formankay JD, Kay LE (1994) A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical-exchange rates of systems in slow equilibrium. J Biomol NMR 4:727–734. doi:10.1007/Bf00404280
Favier A, Brutscher B (2011) Recovering lost magnetization: polarization enhancement in biomolecular NMR. J Biomol NMR 49:9–15. doi:10.1007/s10858-010-9461-5
Fawzi NL, Ying JF, Ghirlando R, Torchia DA, Clore GM (2011) Atomic-resolution dynamics on the surface of amyloid-beta protofibrils probed by solution NMR. Nature 480: 161–268. doi:10.1038/nature10577
Felli IC, Pierattelli R (2015) Spin-state-selective methods in solution- and solid-state biomolecular 13C NMR. Prog Nucl Mag Res Sp 84:1–13. doi:10.1016/j.pnmrs.2014.10.001
Forsen S, Hoffman RA (1963) Study of moderately rapid chemical exchange reactions by means of nuclear magnetic double resonance. J Chem Phys 39:2892–2901. doi:10.1063/1.1734121
Freeman R, Hill HDW (1971) Fourier transform study of NMR spin-lattice relaxation by progressive-saturation. J Chem Phys 54:3367–3377. doi:10.1063/1.1675352
Geen H, Freeman R (1991) Band-selective radiofrequency pulses. J Magn Reson 93:93–141. doi:10.1016/0022-2364(91)90034-Q
Guenneugues M, Berthault P, Desvaux H (1999) A method for determining B1 field inhomogeneity. Are the biases assumed in heteronuclear relaxation experiments usually underestimated? J Magn Reson 136:118–126. doi:10.1006/jmre.1998.1590
Gupta RK, Redfield AG (1970) Double nuclear magnetic resonance observation of electron exchange between ferricytochrome-C and ferrocytochrome-C. Science 169:1204–1206. doi:10.1126/science.169.3951.1204
Henzler-Wildman K, Kern D (2007) Dynamic personalities of proteins. Nature 450:964–972. doi:10.1038/nature06522
Karplus M, Kuriyan J (2005) Molecular dynamics and protein function. Proc Natl Acad Sci USA 102:6679–6685. doi:10.1073/pnas.0408930102
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665. doi:10.1021/ja00052a088
Kimsey IJ, Petzold K, Sathyamoorthy B, Stein ZW, Al-Hashimi HM (2015) Visualizing transient Watson-Crick-like mispairs in DNA and RNA duplexes. Nature 519:315–320. doi:10.1038/nature14227
Kupce E, Freeman R (1993) Polychromatic selective pulses. J Magn Reson Ser A 102:122–126. doi:10.1006/jmra.1993.1079
Lescop E, Schanda P, Brutscher B (2007) A set of BEST triple-resonance experiments for time-optimized protein resonance assignment. J Magn Reson 187:163–169. doi:10.1016/j.jmr.2007.04.002
Lescop E, Kern T, Brutscher B (2010) Guidelines for the use of band-selective radiofrequency pulses in hetero-nuclear NMR: example of longitudinal-relaxation-enhanced BEST-type 1H–15N correlation experiments. J Magn Reson 203:190–198. doi:10.1016/j.jmr.2009.12.001
Meiboom S, Gill D (1958) Modified spin-echo method for measuring nuclear relaxation times. Rev Sci Instrum 29:688–691. doi:10.1063/1.1716296
Mittermaier A, Kay LE (2006) New tools provide new insights in NMR studies of protein dynamics. Science 312:224–228. doi:10.1126/science.1124964
Montelione GT, Wagner G (1989) 2D chemical-exchange NMR-spectroscopy by proton-detected heteronuclear correlation. J Am Chem Soc 111:3096–3098. doi:10.1021/ja00190a072
Mulder FAA, Otten R, Scheek RM (2011) Origin and removal of mixed-phase artifacts in gradient sensitivity enhanced heteronuclear single quantum correlation spectra. J Biomol NMR 51:199–207. doi:10.1007/s10858-011-9554-9
Ottiger M, Delaglio F, Bax A (1998) Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J Magn Reson 131:373–378. doi:10.1006/jmre.1998.1361
Palmer AG, Massi F (2006) Characterization of the dynamics of biomacromolecules using rotating-frame spin relaxation NMR spectroscopy. Chem Rev 106:1700–1719. doi:10.1021/cr0404287
Palmer AG, Kroenke CD, Loria JP (2001) Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Method Enzymol 339:204–238. doi:10.1016/S0076-6879(01)39315-1
Pervushin K, Riek R, Wider G, Wuthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371. doi:10.1073/pnas.94.23.12366
Pervushin KV, Wider G, Wuthrich K (1998) Single transition-to-single transition polarization transfer (ST2-PT) in [15N, 1H]-TROSY. J Biomol NMR 12:345–348. doi:10.1023/A:1008268930690
Pervushin K, Vogeli B, Eletsky A (2002) Longitudinal 1H relaxation optimization in TROSY NMR spectroscopy. J Am Chem Soc 124:12898–12902. doi:10.1021/ja027149q
Schanda P, Van Melckebeke H, Brutscher B (2006) Speeding up three-dimensional protein NMR experiments to a few minutes. J Am Chem Soc 128:9042–9043. doi:10.1021/ja062025p
Schleucher J, Sattler M, Griesinger C (1993) Coherence selection by gradients without signal attenuation: Application to the 3-Dimensional HNCO experiment. Angew Chem Int Edit 32:1489–1491. doi:10.1002/anie.199314891
Sekhar A, Kay LE (2013) NMR paves the way for atomic level descriptions of sparsely populated, transiently formed biomolecular conformers. Proc Natl Acad Sci USA 110:12867–12874. doi:10.1073/pnas.1305688110
Sekhar A, Rosenzweig R, Bouvignies G, Kay LE (2015a) Mapping the conformation of a client protein through the Hsp70 functional cycle. Proc Natl Acad Sci USA 112:10395–10400. doi:10.1073/pnas.1508504112
Sekhar A, Rumfeldt JAO, Broom HR, Doyle CM, Bouvignies G, Meiering EM, Kay LE (2015b) Thermal fluctuations of immature SOD1 lead to separate folding and misfolding pathways. Elife 4:e07296. doi:10.7554/eLife.07296
Sekhar A, Rosenzweig R, Bouvignies G, Kay LE (2016) Hsp70 biases the folding pathways of client proteins. Proc Natl Acad Sci USA 113:E2794–E2801. doi:10.1073/pnas.1601846113
Sklenar V, Torchia D, Bax A (1987) Measurement of 13C longitudinal relaxation using 1H detection. J Magn Reson 73:375–379. doi:10.1016/0022-2364(87)90214-9
Smith MA, Hu H, Shaka AJ (2001) Improved broadband inversion performance for NMR in liquids. J Magn Reson 151:269–283. doi:10.1006/jmre.2001.2364
Tzeng SR, Kalodimos CG (2013) Allosteric inhibition through suppression of transient conformational states. Nat Chem Biol 9:462–465. doi:10.1038/Nchembio.1250
Vallurupalli P, Kay LE (2013) Probing slow chemical exchange at carbonyl sites in proteins by chemical exchange saturation transfer NMR spectroscopy. Angew Chem Int Edit 52:4156–4159. doi:10.1002/anie.201209118
Vallurupalli P, Bouvignies G, Kay LE (2012) Studying “invisible” excited protein states in slow exchange with a major state conformation. J Am Chem Soc 134:8148–8161. doi:10.1021/ja3001419
van Zijl PCM, Yadav NN (2011) Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn Reson Med 65:927–948. doi:10.1002/mrm.22761
Ward KM, Aletras AH, Balaban RS (2000) A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 143:79–87. doi:10.1006/jmre.1999.1956
Wishart DS (2011) Interpreting protein chemical shift data. Prog Nucl Mag Res Sp 58:62–87. doi:10.1016/j.pnmrs.2010.07.004
Yang DW, Kay LE (1999) Improved 1HN-detected triple resonance TROSY-based experiments. J Biomol NMR 13:3–10. doi:10.1023/A:1008329230975
Yang DW, Nagayama K (1996) A sensitivity-enhanced method for measuring heteronuclear long-range coupling constants from the displacement of signals in two 1D subspectra. J Magn Reson Ser A 118:117–121. doi:10.1006/jmra.1996.0017
Yuwen T, Sekhar A, Kay LE (2017) Separating dipolar and chemical exchange magnetization transfer processes in 1H-CEST. Angew Chem Int Edit. doi:10.1002/anie.201610759
Zhao B, Zhang Q (2015) Measuring residual dipolar couplings in excited conformational states of nucleic acids by CEST NMR spectroscopy. J Am Chem Soc 137:13480–13483. doi:10.1021/jacs.5b09014
Zhao B, Hansen AL, Zhang Q (2014) Characterizing slow chemical exchange in nucleic acids by carbon CEST and low spin-lock field R1ρ NMR spectroscopy. J Am Chem Soc 136:20–23. doi:10.1021/ja409835y
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research. L. E. K holds a Canada Research Chair in Biochemistry. Valuable discussions with Dr. Ashok Sekhar are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuwen, T., Kay, L.E. Longitudinal relaxation optimized amide 1H-CEST experiments for studying slow chemical exchange processes in fully protonated proteins. J Biomol NMR 67, 295–307 (2017). https://doi.org/10.1007/s10858-017-0104-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-017-0104-y