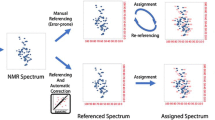
Abstract
Chemical shift prediction has an unappreciated power to guide backbone resonance assignment in cases where protein structure is known. Here we describe Resonance Assignment by chemical Shift Prediction (RASP), a method that exploits this power to derive protein backbone resonance assignments from chemical shift predictions. Robust assignments can be obtained from a minimal set of only the most sensitive triple-resonance experiments, even for spectroscopically challenging proteins. Over a test set of 154 proteins RASP assigns 88 % of residues with an accuracy of 99.7 %, using only information available from HNCO and HNCA spectra. Applied to experimental data from a challenging 34 kDa protein, RASP assigns 90 % of manually assigned residues using only 40 % of the experimental data required for the manual assignment. RASP has the potential to significantly accelerate the backbone assignment process for a wide range of proteins for which structural information is available, including those for which conventional assignment strategies are not feasible.



Similar content being viewed by others
References
Bahrami A, Assadi AH, Markley JL, Eghbalnia HR (2009) Probabilistic interaction network of evidence algorithm and its application to complete labeling of peak lists from protein NMR spectroscopy. PLoS Comput Biol 5:e1000307
Barrett PJ, Chen J, Cho MK, Kim JH, Lu Z, Mathew S, Peng D, Song Y, Van Horn WD, Zhuang T, Sonnichsen FD, Sanders CR (2013) The quiet renaissance of protein nuclear magnetic resonance. Biochemistry 52:1303–1320
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Cavalli A, Salvatella X, Dobson CM, Vendruscolo M (2007) Protein structure determination from NMR chemical shifts. Proc Natl Acad Sci USA 104:9615–9620
Crippen GM, Rousaki A, Revington M, Zhang Y, Zuiderweg ERP (2010) SAGA: rapid automatic mainchain NMR assignment for large proteins. J Biomol NMR 46:281–298
Gronwald W, Willard L, Jellard T, Boyko RF, Rajarathnam K, Wishart DS, Sonnichsen FD, Sykes BD (1998) CAMRA: chemical shift based computer aided protein NMR assignments. J Biomol NMR 12:395–405
Han B, Liu Y, Ginzinger SW, Wishart DS (2011) SHIFTX2: significantly improved protein chemical shift prediction. J Biomol NMR 50:43–57
Headey SJ, Vom A, Simpson JS, Scanlon MJ (2008) Backbone assignments of the 34 kDa ketopantoate reductase from E. coli. Biomol NMR Assign 2:93–96
Jaipuria G, Krishnarjuna B, Mondal S, Dubey A, Atreya HS (2012) Amino acid selective labeling and unlabeling for protein resonance assignments. Adv Exp Med Biol 992:95–118
Jung YS, Zweckstetter M (2004) Mars—robust automatic backbone assignment of proteins. J Biomol NMR 30:11–23
Kohlhoff KJ, Robustelli P, Cavalli A, Salvatella X, Vendruscolo M (2009) Fast and accurate predictions of protein NMR chemical shifts from interatomic distances. J Am Chem Soc 131:13894–13895
Kuhn HW (1955) The Hungarian method for the assignment problem. Naval Res Logist 2:83–87
Langmead CJ, Donald BR (2004) An expectation/maximization nuclear vector replacement algorithm for automated NMR resonance assignments. J Biomol NMR 29:111–138
Lepre CA, Moore JM, Peng JW (2004) Theory and applications of NMR-based screening in pharmaceutical research. Chem Rev 104:3641–3676
MacRaild CA, Anders RF, Foley M, Norton RS (2011) Apical membrane antigen 1 as an anti-malarial drug target. Curr Top Med Chem 11:2039–2047
Matak-Vinkovic D, Vinkovic M, Saldanha SA, Ashurst JL, von Delft F, Inoue T, Miguel RN, Smith AG, Blundell TL, Abell C (2001) Crystal structure of Escherichia coli ketopantoate reductase at 1.7 A resolution and insight into the enzyme mechanism. Biochemistry 40:14493–14500
Mittermaier AK, Kay LE (2009) Observing biological dynamics at atomic resolution using NMR. Trends Biochem Sci 34:601–611
Resende MGC, Ribeiro CC (2010) Greedy randomised adaptive search procedures: advances and applications. In: Gendreau M, Potvin J-Y (eds) Handbook of metaheuristics. Springer, New York, pp 283–319
Richard D, MacRaild CA, Riglar DT, Chan J-A, Foley M, Baum J, Ralph SA, Norton RS, Cowman AF (2010) Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem 285:14815–14822
Schmidt E, Güntert P (2012) A new algorithm for reliable and general NMR resonance assignment. J Am Chem Soc 134:12817–12829
Shen Y, Bax A (2010) SPARTA+: a modest improvement in empirical NMR chemical shift prediction by means of an artificial neural network. J Biomol NMR 48:13–22
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223
Shen Y, Lange O, Delaglio F, Rossi P, Aramini JM, Liu G, Eletsky A, Wu Y, Singarapu KK, Lemak A, Ignatchenko A, Arrowsmith CH, Szyperski T, Montelione GT, Baker D, Bax A (2008) Consistent blind protein structure generation from NMR chemical shift data. Proc Natl Acad Sci USA 105:4685–4690
Stratmann D, Guittet E, van Heijenoort C (2010) Robust structure-based resonance assignment for functional protein studies by NMR. J Biomol NMR 46:157–173
Wishart DS, Arndt D, Berjanskii M, Tang P, Zhou J, Lin G (2008) CS23D: a web server for rapid protein structure generation using NMR chemical shifts and sequence data. Nucleic Acids Res 36:W496–W502
Zhang H, Neal S, Wishart DS (2003) RefDB: a database of uniformly referenced protein chemical shifts. J Biomol NMR 25:173–195
Acknowledgments
We thank Stephen Headey and Martin Scanlon for sharing the KPR data, and David Chalmers for helpful discussions on optimization strategies. This work was supported in part by an Australian National Health and Medical Research Council project grant (1025150). RSN acknowledges fellowship support from the NHMRC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
MacRaild, C.A., Norton, R.S. RASP: rapid and robust backbone chemical shift assignments from protein structure. J Biomol NMR 58, 155–163 (2014). https://doi.org/10.1007/s10858-014-9813-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-014-9813-7