Abstract
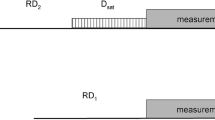
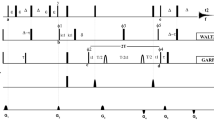
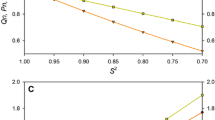
While extracting dynamics parameters from backbone 15N relaxation measurements in proteins has become routine over the past two decades, it is increasingly recognized that accurate quantitative analysis can remain limited by the potential presence of systematic errors associated with the measurement of 15N R1 and R2 or R1ρ relaxation rates as well as heteronuclear 15N-{1H} NOE values. We show that systematic errors in such measurements can be far larger than the statistical error derived from either the observed signal-to-noise ratio, or from the reproducibility of the measurement. Unless special precautions are taken, the problem of systematic errors is shown to be particularly acute in perdeuterated systems, and even more so when TROSY instead of HSQC elements are used to read out the 15N magnetization through the NMR-sensitive 1H nucleus. A discussion of the most common sources of systematic errors is presented, as well as TROSY-based pulse schemes that appear free of systematic errors to the level of <1 %. Application to the small perdeuterated protein GB3, which yields exceptionally high S/N and therefore is an ideal test molecule for detection of systematic errors, yields relaxation rates that show considerably less residue by residue variation than previous measurements. Measured R2′/R1′ ratios fit an axially symmetric diffusion tensor with a Pearson’s correlation coefficient of 0.97, comparable to fits obtained for backbone amide RDCs to the Saupe matrix.






Similar content being viewed by others
References
Bothnerby AA, Shukla R (1988) Spin-locked states of homonuclear 2-spin systems. J Magn Reson 77:524–535
Boyd J, Hommel U, Campbell ID (1990) Influence of cross-correlation between dipolar and anisotropic chemical shift relaxation mechanisms upon longitudinal relaxation rates of 15 N in macromolecules. Chem Phys Lett 175:477–482
Burum DP, Ernst RR (1980) Net polarization transfer via a J-ordered state for signal enhancement of low-sensitivity nuclei. J Magn Reson 39:163–168
Cavanagh J, Fairbrother WJ, Palmer AG, Rance M, Skelton N (2007) Protein NMR spectroscopy: principles and practice. Elsevier Academic Press, Burlington
Chen K, Tjandra N (2011) Water proton spin saturation affects measured protein backbone (15)N spin relaxation rates. J Magn Reson 213:151–157
Chiarparin E, Pelupessy P, Ghose R, Bodenhausen G (1999) Relaxation of two-spin coherence due to cross-correlated fluctuations of dipole–dipole couplings and anisotropic shifts in NMR of N-15,C-13-labeled biomolecules. J Am Chem Soc 121:6876–6883
Chill JH, Louis JM, Baber JL, Bax A (2006) Measurement of N-15 relaxation in the detergent-solubilized tetrameric KcsA potassium channel. J Biomol NMR 36:123–136
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRpipe—a multidimensional spectral processing system based on unix pipes. J Biomol NMR 6:277–293
Farrow NA, Muhandiram R, Singer AU, Pascal SM, Kay CM, Gish G, Shoelson SE, Pawson T, Forman-Kay JD, Kay LE (1994) Backbone dynamics of a free and a phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33:5984–6003
Favier A, Brutscher B (2011) Recovering lost magnetization: polarization enhancement in biomolecular NMR. J Biomol NMR 49:9–15
Felli IC, Desvaux H, Bodenhausen G (1998) Local mobility of N-15 labeled biomolecules characterized through cross-correlation rates: applications to paramagnetic proteins. J Biomol NMR 12:509–521
Ferrage F, Piserchio A, Cowburn D, Ghose R (2008) On the measurement of N-15-{H-1} nuclear Overhauser effects. J Magn Reson 192:302–313
Ferrage F, Cowburn D, Ghose R (2009) Accurate sampling of high-frequency motions in proteins by steady-state (15)N-{(1)H} nuclear Overhauser effect measurements in the presence of cross-correlated relaxation. J Am Chem Soc 131: 6048–+
Ferrage F, Reichel A, Battacharya S, Cowburn D, Ghose R (2010) On the measurement of (15)N-{(1)H} nuclear Overhauser effects. 2. Effects of the saturation scheme and water signal suppression. J Magn Reson 207:294–303
Freeman R, Kempsell SP, Levitt MH (1980) Radiofrequency pulse sequences which compensate their own imperfections. J Magn Reson 38:453–479
Fushman D, Cowburn D (1998) Model-independent analysis of N-15 chemical shift anisotropy from NMR relaxation data. Ubiquitin as a test example. J Am Chem Soc 120:7109–7110
Fushman D, Cowburn D (1999) The effect of noncollinearity of N-15-H-1 dipolar and N-15 CSA tensors and rotational anisotropy on N-15 relaxation, CSA/dipolar cross correlation, and TROSY. J Biomol NMR 13:139–147
Fushman D, Tjandra N, Cowburn D (1998) Direct measurement of N-15 chemical shift anisotropy in solution. J Am Chem Soc 120:10947–10952
Fushman D, Xu R, Cowburn D (1999) Direct determination of changes of interdomain orientation on ligation: use of the orientational dependence of N-15 NMR relaxation in Abl SH(32). Biochemistry 38:10225–10230
Garwood M, Ke Y (1991) Symmetrical pulses to induce arbitrary flip angles with compensation for RF inhomogeneity and resonance offsets. J Magn Reson 94:511–525
Geen H, Freeman R (1991) Band-selective radiofrequency pulses. J Magn Reson 93:93–141
Grzesiek S, Bax A (1993) The importance of not saturating H2O in protein NMR. Application to sensitivity enhancement and NOE measurement. J Am Chem Soc 115:12593
Hall JB, Fushman D (2003) Characterization of the overall and local dynamics of a protein with intermediate rotational anisotropy: differentiating between conformational exchange and anisotropic diffusion in the B3 domain of protein G. J Biomol NMR 27:261–275
Hall JB, Fushman D (2006) Variability of the N-15 chemical shielding tensors in the B3 domain of protein G from N-15 relaxation measurements at several fields. Implications for backbone order parameters. J Am Chem Soc 128:7855–7870
Hansen DF, Kay LE (2007) Improved magnetization alignment schemes for spin-lock relaxation experiments. J Biomol NMR 37:245–255
Hare BJ, Wyss DF, Osburne MS, Kern PS, Reinherz EL, Wagner G (1999) Structure, specificity and CDR mobility of a class II restricted single-chain T-cell receptor. Nat Struct Biol 6:574–581
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple amber force fields and development of improved protein backbone parameters. Prot Struct Funct Bioinform 65:712–725
Ishima R, Torchia DA (2000) Protein dynamics from NMR. Nat Struct Biol 7:740–743
Kay LE (1998) Protein dynamics from NMR. Nat Struct Biol 513–517
Kay LE, Torchia DA, Bax A (1989) Backbone dynamics of proteins as studied by 15 N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry 28:8972–8979
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Kern D, Zuiderweg ERP (2003) The role of dynamics in allosteric regulation. Curr Opin Struct Biol 13:748–757
Kobzar K, Skinner TE, Khaneja N, Glaser SJ, Luy B (2008) Exploring the limits of broadband excitation and inversion: II. Rf-power optimized pulses. J Magn Reson 194:58–66
Korzhnev DM, Skrynnikov NR, Millet O, Torchia DA, Kay LE (2002) An NMR experiment for the accurate measurement of heteronuclear spin-lock relaxation rates. J Am Chem Soc 124:10743–10753
Lee AL, Wand AJ (2001) Microscopic origins of entropy, heat capacity and the glass transition in proteins. Nature 411:501–504
Lee AL, Kinnear SA, Wand AJ (2000) Redistribution and loss of side chain entropy upon formation of a calmodulin-peptide complex. Nat Struct Biol 7:72–77
Li D-W, Brueschweiler R (2009) A dictionary for protein side-chain entropies from NMR order parameters. J Am Chem Soc 131:7226–7227
Markley JL, Horsley WJ, Klein MP (1971) Spin-lattice relaxation measurements in slowly relaxing complex spectra. J Chem Phys 55:3604–3605
Massi F, Johnson E, Wang CY, Rance M, Palmer AG (2004) NMR R-1 rho rotating-frame relaxation with weak radio frequency fields. J Am Chem Soc 126:2247–2256
Millet O, Muhandiram DR, Skrynnikov NR, Kay LE (2002) Deuterium spin probes of side-chain dynamics in proteins. 1. Measurement of five relaxation rates per deuteron in C-13-labeled and fractionally H-2-enriched proteins in solution. J Am Chem Soc 124:6439–6448
Mitschang L, Keeler J, Davis AL, Oschkinat H (1992) Removal of zero-quantum interference in NOESY spectra of proteins by utilizing the natural inhomogeneity of the radiofrequency field. J Biomol NMR 2:545–556
Mittag T, Kay LE, Forman-Kay JD (2010) Protein dynamics and conformational disorder in molecular recognition. J Mol Recognit 23:105–116
Mittermaier A, Kay LE (2006) Review—new tools provide new insights in NMR studies of protein dynamics. Science 312:224–228
Muhandiram DR, Yamaaki T, Sykes BD, Kay LE (1995) Measurement of 2H T1 and T1p relaxation times in uniformly 13C-labeled and fractionally 2H-labeled proteins in solution. J Am Chem Soc 117:11536–11544
Mulder FAA, de Graaf RA, Kaptein R, Boelens R (1998) An off-resonance rotating frame relaxation experiment for the investigation of macromolecular dynamics using adiabatic rotations. J Magn Reson 131:351–357
Nadaud PS, Helmus JJ, Jaroniec CP (2007) 13C and 15 N chemical shift assignments and secondary structure of the B3 immunoglobulin-binding domain of streptococcal protein G by magic-angle spinning solid-state NMR spectroscopy. Biomol NMR Assign 1:117–120
Namanja AT, Wang XJ, Xu B, Mercedes-Camacho AY, Wilson KA, Etzkorn FA, Peng JW (2011) Stereospecific gating of functional motions in Pin1. Proc Natl Acad Sci USA 108:12289–12294
Nietlispach D (2005) Suppression of anti-TROSY lines in a sensitivity enhanced gradient selection TROSY scheme. J Biomol NMR 31:161–166
Nirmala NR, Wagner G (1988) Measurement Of C-13 relaxation-times in proteins by two-dimensional heteronuclear H1-C-13 correlation spectroscopy. J Am Chem Soc 110:7557–7558
Palmer AG (2004) NMR characterization of the dynamics of biomacromolecules. Chem Rev (Washington, DC, US) 104:3623–3640
Pelupessy P, Chiarparin E, Ghose R, Bodenhausen G (1999) Simultaneous determination of Psi and Phi angles in proteins from measurements of cross-correlated relaxation effects. J Biomol NMR 14:277–280
Peng JW, Wagner G (1992) Mapping of spectral density functions using heteronuclear NMR relaxation measurements. J Magn Reson 98:308–332
Peng JW, Thanabal V, Wagner G (1991) 2d heteronuclear NMR measurements of spin-lattice relaxation-times in the rotating frame of X nuclei in heteronuclear HX spin systems. J Magn Reson 94:82–100
Pervushin K, Riek R, Wider G, Wuthrich K (1997) Attenuated T-2 relaxation by mutual cancellation of dipole–dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371
Pervushin KV, Wider G, Wuthrich K (1998) Single transition-to-single transition polarization transfer (ST2-PT) in [N15, H1]-TROSY. J Biomol NMR 12:345–348
Piotto M, Saudek V, Sklenár V (1992) Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR 2:661–665
Popovych N, Sun S, Ebright RH, Kalodimos CG (2006) Dynamically driven protein allostery. Nat Struct Mol Biol 13:831–838
Price DJ, Brooks CL (2002) Modern protein force fields behave comparably in molecular dynamics simulations. J Comput Chem 23:1045–1057
Reif B, Hennig M, Griesinger C (1997) Direct measurement of angles between bond vectors in high-resolution NMR. Science 276:1230–1233
Rinnenthal J, Buck J, Ferner J, Wacker A, Fuertig B, Schwalbe H (2011) Mapping the landscape of RNA dynamics with NMR spectroscopy. Acc Chem Res 44:1292–1301
Showalter SA, Bruschweiler R (2007) Validation of molecular dynamics simulations of biomolecules using NMR spin relaxation as benchmarks: application to the AMBER99SB force field. J Chem Theory Comput 3:961–975
Sklenar V, Torchia D, Bax A (1987) Measurement of C-13 longitudinal relaxation using H-1 detection. J Magn Reson 73:375–379
Smith MA, Hu H, Shaka AJ (2001) Improved broadband inversion performance for NMR in liquids. J Magn Reson 151:269–283
Stocker U, van Gunsteren WF (2000) Molecular dynamics simulation of hen egg white lysozyme: a test of the GROMOS96 force field against nuclear magnetic resonance data. Prot Struct Funct Genet 40:145–153
Stone MJ (2001) NMR relaxation studies of the role of conformational entropy in protein stability and ligand binding. Acc Chem Res 34:379–388
Tjandra N, Feller SE, Pastor RW, Bax A (1995) Rotational diffusion anisotropy of human ubiquitin from N-15 NMR relaxation. J Am Chem Soc 117:12562–12566
Tjandra N, Szabo A, Bax A (1996) Protein backbone dynamics and N-15 chemical shift anisotropy from quantitative measurement of relaxation interference effects. J Am Chem Soc 118:6986–6991
Ulmer TS, Campbell ID, Boyd J (2004) Amide proton relaxation measurements employing a highly deuterated protein. J Magn Reson 166:190–201
Varadan R, Assfalg M, Haririnia A, Raasi S, Pickart C, Fushman D (2004) Solution conformation of Lys(63)-linked di-ubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J Biol Chem 279:7055–7063
Walker O, Varadan R, Fushman D (2004) Efficient and accurate determination of the overall rotational diffusion tensor of a molecule from N-15 relaxation data using computer program ROTDIF. J Magn Reson 168:336–345
Wang AC, Bax A (1993) Minimizing the effects of radiofrequency heating in multidimensional NMR experiments. J Biomol NMR 3:715–720
Yang DW, Mok YK, FormanKay JD, Farrow NA, Kay LE (1997) Contributions to protein entropy and heat capacity from bond vector motions measured by NMR spin relaxation. J Mol Biol 272:790–804
Yang DW, Mittermaier A, Mok YK, Kay LE (1998) A study of protein side-chain dynamics from new H-2 auto- correlation and C-13 cross-correlation NMR experiments: application to the N-terminal SH3 domain from drk. J Mol Biol 276:939–954
Zhu G, Xia YL, Nicholson LK, Sze KH (2000) Protein dynamics measurements by TROSY-based NMR experiments. J Magn Reson 143:423–426
Zidek L, Novotny MV, Stone MJ (1999) Increased protein backbone conformational entropy upon hydrophobic ligand binding. Nat Struct Biol 6:1118–1121
Acknowledgments
We thank Dennis A Torchia and Alex Grishaev for helpful discussions and computer simulations, Frank Delaglio for assistance with the evaluation of relaxation data and Alex Maltsev for help with the expression and purification of GB3. This work was funded by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH) and the Intramural AIDS-Targeted Antiviral Program of the Office of the Director, NIH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lakomek, NA., Ying, J. & Bax, A. Measurement of 15N relaxation rates in perdeuterated proteins by TROSY-based methods. J Biomol NMR 53, 209–221 (2012). https://doi.org/10.1007/s10858-012-9626-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-012-9626-5